
PDF Publication Title:
Text from PDF Page: 003
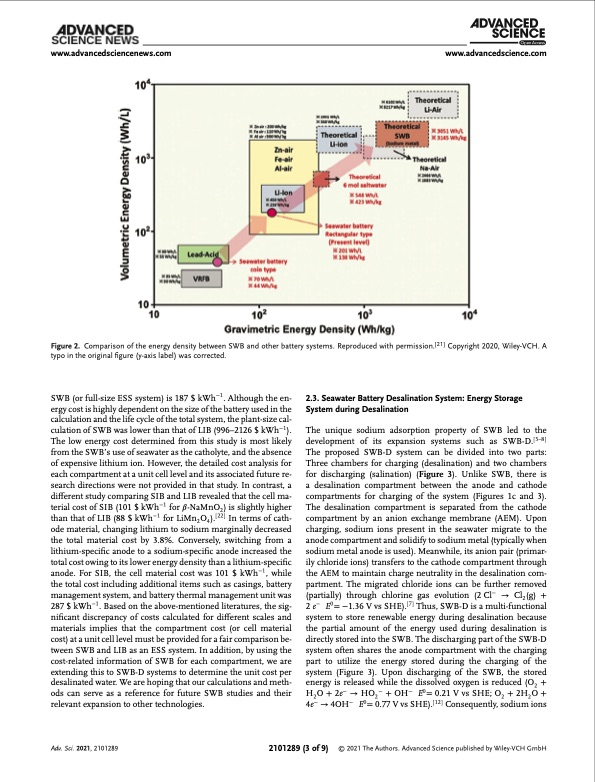
www.advancedsciencenews.com www.advancedscience.com Figure 2. Comparison of the energy density between SWB and other battery systems. Reproduced with permission.[21] Copyright 2020, Wiley-VCH. A typo in the original figure (y-axis label) was corrected. SWB (or full-size ESS system) is 187 $ kWh−1. Although the en- ergy cost is highly dependent on the size of the battery used in the calculation and the life cycle of the total system, the plant-size cal- culation of SWB was lower than that of LIB (996–2126 $ kWh−1). The low energy cost determined from this study is most likely from the SWB’s use of seawater as the catholyte, and the absence of expensive lithium ion. However, the detailed cost analysis for each compartment at a unit cell level and its associated future re- search directions were not provided in that study. In contrast, a different study comparing SIB and LIB revealed that the cell ma- terial cost of SIB (101 $ kWh−1 for 𝛽-NaMnO2) is slightly higher than that of LIB (88 $ kWh−1 for LiMn2 O4 ).[ 22 ] In terms of cath- ode material, changing lithium to sodium marginally decreased the total material cost by 3.8%. Conversely, switching from a lithium-specific anode to a sodium-specific anode increased the total cost owing to its lower energy density than a lithium-specific anode. For SIB, the cell material cost was 101 $ kWh−1, while the total cost including additional items such as casings, battery management system, and battery thermal management unit was 287 $ kWh−1. Based on the above-mentioned literatures, the sig- nificant discrepancy of costs calculated for different scales and materials implies that the compartment cost (or cell material cost) at a unit cell level must be provided for a fair comparison be- tween SWB and LIB as an ESS system. In addition, by using the cost-related information of SWB for each compartment, we are extending this to SWB-D systems to determine the unit cost per desalinated water. We are hoping that our calculations and meth- ods can serve as a reference for future SWB studies and their relevant expansion to other technologies. 2.3. Seawater Battery Desalination System: Energy Storage System during Desalination The unique sodium adsorption property of SWB led to the development of its expansion systems such as SWB-D.[ 5–8 ] The proposed SWB-D system can be divided into two parts: Three chambers for charging (desalination) and two chambers for discharging (salination) (Figure 3). Unlike SWB, there is a desalination compartment between the anode and cathode compartments for charging of the system (Figures 1c and 3). The desalination compartment is separated from the cathode compartment by an anion exchange membrane (AEM). Upon charging, sodium ions present in the seawater migrate to the anode compartment and solidify to sodium metal (typically when sodium metal anode is used). Meanwhile, its anion pair (primar- ily chloride ions) transfers to the cathode compartment through the AEM to maintain charge neutrality in the desalination com- partment. The migrated chloride ions can be further removed (partially) through chlorine gas evolution (2 Cl− → Cl2 (g) + 2 e− E0 = −1.36 V vs SHE).[ 7 ] Thus, SWB-D is a multi-functional system to store renewable energy during desalination because the partial amount of the energy used during desalination is directly stored into the SWB. The discharging part of the SWB-D system often shares the anode compartment with the charging part to utilize the energy stored during the charging of the system (Figure 3). Upon discharging of the SWB, the stored energy is released while the dissolved oxygen is reduced (O2 + H2O+2e− →HO2− +OH− E0=0.21VvsSHE;O2 +2H2O+ 4e− → 4OH− E0 = 0.77 V vs SHE).[ 12 ] Consequently, sodium ions Adv. Sci. 2021, 2101289 2101289 (3 of 9) © 2021 The Authors. Advanced Science published by Wiley-VCH GmbHPDF Image | Seawater Desalination using Rechargeable Seawater Battery
PDF Search Title:
Seawater Desalination using Rechargeable Seawater BatteryOriginal File Name Searched:
advs2827.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |