
PDF Publication Title:
Text from PDF Page: 004
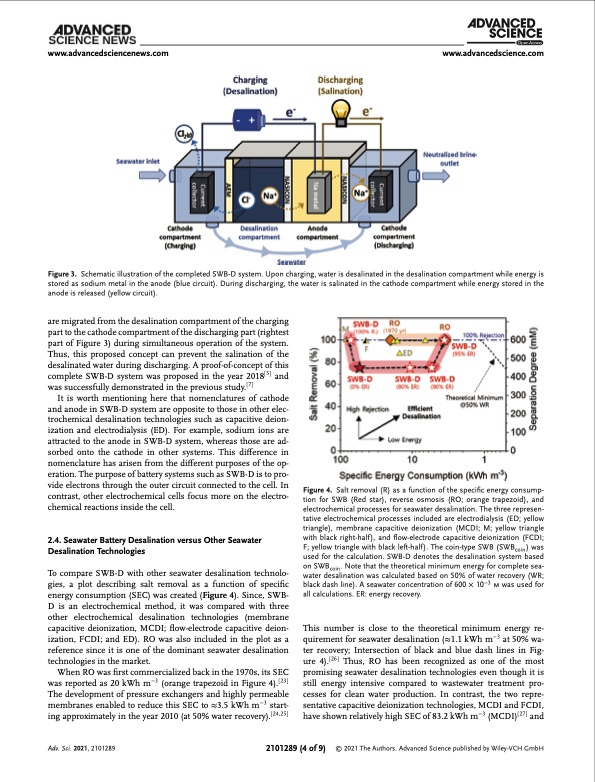
www.advancedsciencenews.com www.advancedscience.com Figure 3. Schematic illustration of the completed SWB-D system. Upon charging, water is desalinated in the desalination compartment while energy is stored as sodium metal in the anode (blue circuit). During discharging, the water is salinated in the cathode compartment while energy stored in the anode is released (yellow circuit). are migrated from the desalination compartment of the charging part to the cathode compartment of the discharging part (rightest part of Figure 3) during simultaneous operation of the system. Thus, this proposed concept can prevent the salination of the desalinated water during discharging. A proof-of-concept of this complete SWB-D system was proposed in the year 2018[5] and was successfully demonstrated in the previous study.[7] It is worth mentioning here that nomenclatures of cathode and anode in SWB-D system are opposite to those in other elec- trochemical desalination technologies such as capacitive deion- ization and electrodialysis (ED). For example, sodium ions are attracted to the anode in SWB-D system, whereas those are ad- sorbed onto the cathode in other systems. This difference in nomenclature has arisen from the different purposes of the op- eration. The purpose of battery systems such as SWB-D is to pro- vide electrons through the outer circuit connected to the cell. In contrast, other electrochemical cells focus more on the electro- chemical reactions inside the cell. 2.4. Seawater Battery Desalination versus Other Seawater Desalination Technologies To compare SWB-D with other seawater desalination technolo- gies, a plot describing salt removal as a function of specific energy consumption (SEC) was created (Figure 4). Since, SWB- D is an electrochemical method, it was compared with three other electrochemical desalination technologies (membrane capacitive deionization, MCDI; flow-electrode capacitive deion- ization, FCDI; and ED). RO was also included in the plot as a reference since it is one of the dominant seawater desalination technologies in the market. When RO was first commercialized back in the 1970s, its SEC was reported as 20 kWh m−3 (orange trapezoid in Figure 4).[ 23 ] The development of pressure exchangers and highly permeable membranes enabled to reduce this SEC to ≈3.5 kWh m−3 start- ing approximately in the year 2010 (at 50% water recovery).[ 24,25 ] Figure 4. Salt removal (R) as a function of the specific energy consump- tion for SWB (Red star), reverse osmosis (RO; orange trapezoid), and electrochemical processes for seawater desalination. The three represen- tative electrochemical processes included are electrodialysis (ED; yellow triangle), membrane capacitive deionization (MCDI; M; yellow triangle with black right-half), and flow-electrode capacitive deionization (FCDI; F; yellow triangle with black left-half). The coin-type SWB (SWBcoin) was used for the calculation. SWB-D denotes the desalination system based on SWBcoin. Note that the theoretical minimum energy for complete sea- water desalination was calculated based on 50% of water recovery (WR; black dash line). A seawater concentration of 600 × 10−3 m was used for all calculations. ER: energy recovery. This number is close to the theoretical minimum energy re- quirement for seawater desalination (≈1.1 kWh m−3 at 50% wa- ter recovery; Intersection of black and blue dash lines in Fig- ure 4).[26] Thus, RO has been recognized as one of the most promising seawater desalination technologies even though it is still energy intensive compared to wastewater treatment pro- cesses for clean water production. In contrast, the two repre- sentative capacitive deionization technologies, MCDI and FCDI, have shown relatively high SEC of 83.2 kWh m−3 (MCDI)[ 27 ] and Adv. Sci. 2021, 2101289 2101289 (4 of 9) © 2021 The Authors. Advanced Science published by Wiley-VCH GmbHPDF Image | Seawater Desalination using Rechargeable Seawater Battery
PDF Search Title:
Seawater Desalination using Rechargeable Seawater BatteryOriginal File Name Searched:
advs2827.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |