
PDF Publication Title:
Text from PDF Page: 002
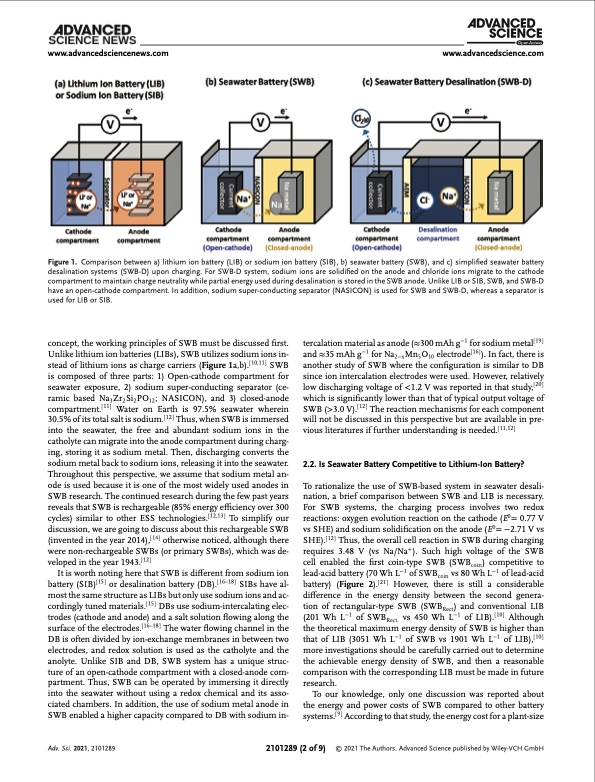
www.advancedsciencenews.com www.advancedscience.com Figure 1. Comparison between a) lithium ion battery (LIB) or sodium ion battery (SIB), b) seawater battery (SWB), and c) simplified seawater battery desalination systems (SWB-D) upon charging. For SWB-D system, sodium ions are solidified on the anode and chloride ions migrate to the cathode compartment to maintain charge neutrality while partial energy used during desalination is stored in the SWB anode. Unlike LIB or SIB, SWB, and SWB-D have an open-cathode compartment. In addition, sodium super-conducting separator (NASICON) is used for SWB and SWB-D, whereas a separator is used for LIB or SIB. concept, the working principles of SWB must be discussed first. Unlike lithium ion batteries (LIBs), SWB utilizes sodium ions in- stead of lithium ions as charge carriers (Figure 1a,b).[ 10,11 ] SWB is composed of three parts: 1) Open-cathode compartment for seawater exposure, 2) sodium super-conducting separator (ce- ramic based Na3 Zr2 Si2 PO12 ; NASICON), and 3) closed-anode compartment.[11] Water on Earth is 97.5% seawater wherein 30.5% of its total salt is sodium.[ 12 ] Thus, when SWB is immersed into the seawater, the free and abundant sodium ions in the catholyte can migrate into the anode compartment during charg- ing, storing it as sodium metal. Then, discharging converts the sodium metal back to sodium ions, releasing it into the seawater. Throughout this perspective, we assume that sodium metal an- ode is used because it is one of the most widely used anodes in SWB research. The continued research during the few past years reveals that SWB is rechargeable (85% energy efficiency over 300 cycles) similar to other ESS technologies.[ 12,13 ] To simplify our discussion, we are going to discuss about this rechargeable SWB (invented in the year 2014),[ 14 ] otherwise noticed, although there were non-rechargeable SWBs (or primary SWBs), which was de- veloped in the year 1943.[12] It is worth noting here that SWB is different from sodium ion battery (SIB)[ 15 ] or desalination battery (DB).[ 16–18 ] SIBs have al- most the same structure as LIBs but only use sodium ions and ac- cordingly tuned materials.[ 15 ] DBs use sodium-intercalating elec- trodes (cathode and anode) and a salt solution flowing along the surface of the electrodes.[ 16–18 ] The water flowing channel in the DB is often divided by ion-exchange membranes in between two electrodes, and redox solution is used as the catholyte and the anolyte. Unlike SIB and DB, SWB system has a unique struc- ture of an open-cathode compartment with a closed-anode com- partment. Thus, SWB can be operated by immersing it directly into the seawater without using a redox chemical and its asso- ciated chambers. In addition, the use of sodium metal anode in SWB enabled a higher capacity compared to DB with sodium in- tercalation material as anode (≈300 mAh g−1 for sodium metal[ 19 ] and ≈35 mAh g−1 for Na2−x Mn5 O10 electrode[ 16 ] ). In fact, there is another study of SWB where the configuration is similar to DB since ion intercalation electrodes were used. However, relatively low discharging voltage of <1.2 V was reported in that study,[20] which is significantly lower than that of typical output voltage of SWB (>3.0 V).[ 12 ] The reaction mechanisms for each component will not be discussed in this perspective but are available in pre- vious literatures if further understanding is needed.[ 11,12 ] 2.2. Is Seawater Battery Competitive to Lithium-Ion Battery? To rationalize the use of SWB-based system in seawater desali- nation, a brief comparison between SWB and LIB is necessary. For SWB systems, the charging process involves two redox reactions: oxygen evolution reaction on the cathode (E0= 0.77 V vs SHE) and sodium solidification on the anode (E0= −2.71 V vs SHE).[ 12 ] Thus, the overall cell reaction in SWB during charging requires 3.48 V (vs Na/Na+). Such high voltage of the SWB cell enabled the first coin-type SWB (SWBcoin) competitive to lead-acid battery (70 Wh L−1 of SWBcoin vs 80 Wh L−1 of lead-acid battery) (Figure 2).[ 21 ] However, there is still a considerable difference in the energy density between the second genera- tion of rectangular-type SWB (SWBRect) and conventional LIB (201 Wh L−1 of SWBRect. vs 450 Wh L−1 of LIB).[10] Although the theoretical maximum energy density of SWB is higher than that of LIB (3051 Wh L−1 of SWB vs 1901 Wh L−1 of LIB),[10] more investigations should be carefully carried out to determine the achievable energy density of SWB, and then a reasonable comparison with the corresponding LIB must be made in future research. To our knowledge, only one discussion was reported about the energy and power costs of SWB compared to other battery systems.[ 9 ] According to that study, the energy cost for a plant-size Adv. Sci. 2021, 2101289 2101289 (2 of 9) © 2021 The Authors. Advanced Science published by Wiley-VCH GmbHPDF Image | Seawater Desalination using Rechargeable Seawater Battery
PDF Search Title:
Seawater Desalination using Rechargeable Seawater BatteryOriginal File Name Searched:
advs2827.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |