
PDF Publication Title:
Text from PDF Page: 003
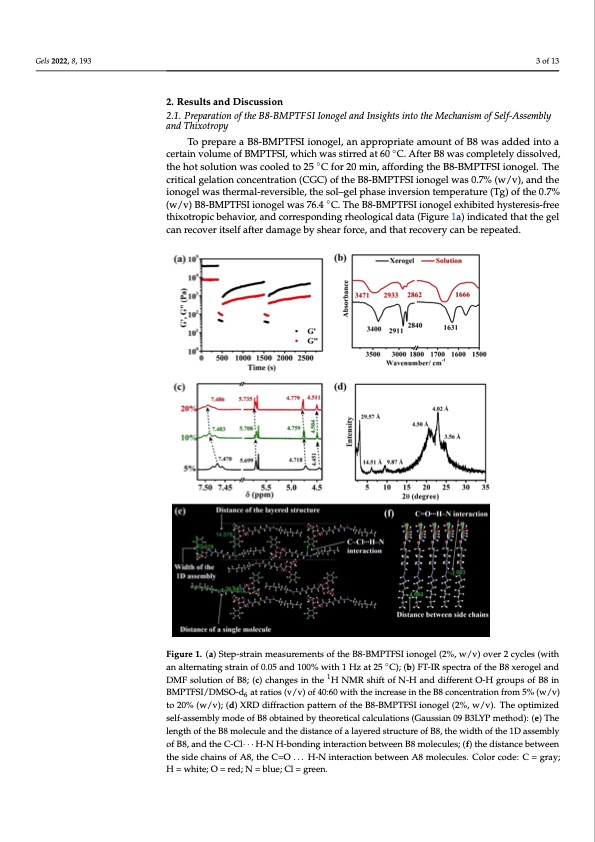
Gels 2022, 8, 193 3 of 13 2. Results and Discussion 2.1. Preparation of the B8-BMPTFSI Ionogel and Insights into the Mechanism of Self-Assembly and Thixotropy To prepare a B8-BMPTFSI ionogel, an appropriate amount of B8 was added into a certain volume of BMPTFSI, which was stirred at 60 ◦C. After B8 was completely dissolved, the hot solution was cooled to 25 ◦C for 20 min, affording the B8-BMPTFSI ionogel. The critical gelation concentration (CGC) of the B8-BMPTFSI ionogel was 0.7% (w/v), and the ionogel was thermal-reversible, the sol–gel phase inversion temperature (Tg) of the 0.7% (w/v) B8-BMPTFSI ionogel was 76.4 ◦C. The B8-BMPTFSI ionogel exhibited hysteresis-free thixotropic behavior, and corresponding rheological data (Figure 1a) indicated that the gel Gels 2022, 8, x FOR PEER REVIEW 4 of 14 can recover itself after damage by shear force, and that recovery can be repeated. Figure 1. (a) Step-strain measurements of the B8-BMPTFSI ionogel (2%, w/v) over 2 cycles (with an Figure 1. (a) Staelpte-rsntartainignsmtraeinasouf 0r.e0m5 aenndt1s00o%f twhiethB18H-zBaMt 2P5T°CF)S; (Ibi)oFnTo-IgReslpe(2ct%ra,owf th/evB)8oxvereorge2l acnydclDeMs F(with solution of B8; (c) changes in the 1H NMR shi◦ft of N-H and different O-H groups of B8 in an alternating strain of 0.05 and 100% with 1 Hz at 25 C); (b) FT-IR spectra of the B8 xerogel and BMPTFSI/DMSO-d6 at ratios (v/v) of 40:60 with the increase in the B8 concentration from 5% (w/v) DMF solution of B8; (c) changes in the 1H NMR shift of N-H and different O-H groups of B8 in to 20% (w/v); (d) XRD diffraction pattern of the B8-BMPTFSI ionogel (2%, w/v). The optimized self- BMPTFSI/DMSaOss-edmblaytmraotdioesof(vB/8vo)btoafin4e0d:6b0y wtheitohrethicealicnaclcruelastieonins (tGhaeusBs8ianco0n9cBe3nLtYrPatmioenthfordo)m: (e5)%Th(ew/v) 6 length of the B8 molecule and the distance of a layered structure of B8, the width of the 1D assembly to 20% (w/v); (d) XRD diffraction pattern of the B8-BMPTFSI ionogel (2%, w/v). The optimized of B8, and the C-Cl‧‧‧H-N H-bonding interaction between B8 molecules; (f) the distance between the self-assembly mode of B8 obtained by theoretical calculations (Gaussian 09 B3LYP method): (e) The side chains of A8, the C=O...H-N interaction between A8 molecules. Color code: C = gray; H = white; length of the B8Om=oreledc; Nul=ebalnued; Cthl =egdriesetna.nce of a layered structure of B8, the width of the 1D assembly of B8, and the C-Cl· · · H-N H-bonding interaction between B8 molecules; (f) the distance between X-ray diffraction (XRD) patterns of the B8-BMPTFSI ionogel (Figure 1d) were rec- the side chains of A8, the C=O . . . H-N interaction between A8 molecules. Color code: C = gray; orded to obtain information regarding the self-assembly of B8. Three clear reflection peaks H = white; O = red; N = blue; Cl = green. corresponding to d-spacings of 29.57 Å, 14.51 Å, and 9.87 Å in a ratio of 1: 1/2: 1/3 were observed, respectively, suggesting the presence of a lamellar structure with a periodicity of 29.57 Å in the gel state [35]. The peak corresponding to a d-spacing of 3.56 Å is charac- teristic of the π-π stacking distance, indicative of the presence of π-π interactions betweenPDF Image | Thixotropic Ionogel Electrolyte for Sodium Batteries
PDF Search Title:
Thixotropic Ionogel Electrolyte for Sodium BatteriesOriginal File Name Searched:
gels-08-00193-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |