
PDF Publication Title:
Text from PDF Page: 002
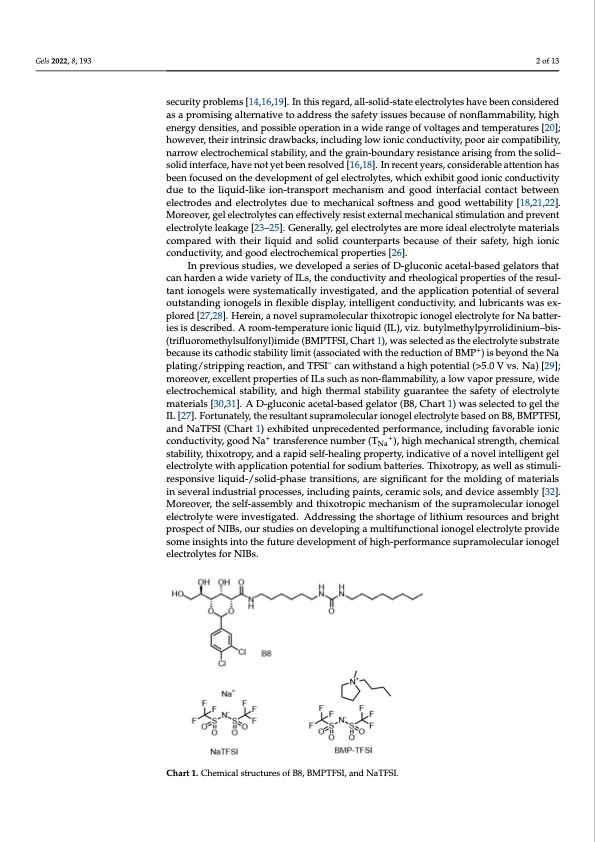
Gels 2022, 8, x FOR PEER REVIEW Gels 2022, 8, 193 2 of 14 2 of 13 conductivity, a wide electrochemical stability window, a relatively higher capacity, and a stable anode-passivation layer; however, their flammability and high vapor pressure lead to endless security problems [14,16,19]. In this regard, all-solid-state electrolytes have been security problems [14,16,19]. In this regard, all-solid-state electrolytes have been considered considered as a promising alternative to address the safety issues because of nonflamma- as a promising alternative to address the safety issues because of nonflammability, high bility, high energy densities, and possible operation in a wide range of voltages and tem- energy densities, and possible operation in a wide range of voltages and temperatures [20]; peratures [20]; however, their intrinsic drawbacks, including low ionic conductivity, poor however, their intrinsic drawbacks, including low ionic conductivity, poor air compatibility, air compatibility, narrow electrochemical stability, and the grain-boundary resistance narrow electrochemical stability, and the grain-boundary resistance arising from the solid– arising from the solid–solid interface, have not yet been resolved [16,18]. In recent years, solid interface, have not yet been resolved [16,18]. In recent years, considerable attention has considerable attention has been focused on the development of gel electrolytes, which ex- been focused on the development of gel electrolytes, which exhibit good ionic conductivity hibit good ionic conductivity due to the liquid-like ion-transport mechanism and good due to the liquid-like ion-transport mechanism and good interfacial contact between interfacial contact between electrodes and electrolytes due to mechanical softness and electrodes and electrolytes due to mechanical softness and good wettability [18,21,22]. good wettability [18,21,22]. Moreover, gel electrolytes can effectively resist external me- Moreover, gel electrolytes can effectively resist external mechanical stimulation and prevent chanical stimulation and prevent electrolyte leakage [23–25]. Generally, gel electrolytes electrolyte leakage [23–25]. Generally, gel electrolytes are more ideal electrolyte materials caormepmaorerde iwdeitahl ethlecitrrolilqyuteidmantedrisaolslidcocmopuanrtedrpwaritshbtheceairusliequoifdthaenidr saofleidty,cohuignhteriponaricts cboencdaucsteivoiftyt,haenirdsgafoeotdy,ehleigchtroiocnhiecmciocnadl uprcotipveitryti,easn[d26g]o. od electrochemical properties [26]. IInnpprreevvioiouusssstutuddieiess, ,weeddeevveeloloppeeddaasseerireiessoof fDD-g-gluluccoonnicicaacceetatal-lb-baasseeddggeelalatotorrssththaatt ccaannhhaarrddeennaawiidevarriietty of ILs, the conductivity and rheological properties of the resullt-- tant ionogellsweerreessyystsetemmaatitciacalllylyinivnevsetsigtiagtaetde,da,nadntdhethaeppaplipcalitcioantiopnotpeonteianltoiaflsoevf esreavleoruatl- osutatsntdanindginiogniogneolsgeinlsflienxflibelxeidbilsepdlaisyp,lianyt,elilnigtelnlitgceonntdcuocntdivuitcyti,vaintyd,launbdrilcuabnrtiscwanatssewxpalsoerexd- p[l2o7r,e2d8][.2H7,e2r8e].inH,earneionv,ealnsouvperlasmuoplreacmuloalrectuhliaxrotrhoixpoictriopniocgioenloegleeclterolelcytreoflyotreNfoarbNaattebraitetseri-s iedseisscrdibesecdr.ibAedro. oAmro-toem-ptemraptuereatiuorneiciolniqicuildiqu(IiLd),(IvLi)z,.vbiuz.tyblumtyeltmhyeltphyrlrpoylridroinliiduimni–ubmis–-b(tirsi-- (tfrluiflourormomethetyhlysulslufolfnoynly)ilm)imidiede(B(BMMPPTTFFSSI,I,CChhaarrtt11)),,wasssellected as the electrollyttessubbssttrraatete because its cathodic stability limit (associated with the reduction of BMP+) is beyond the Na because its cathodic stability limit (associated with the reduction of BMP+) is beyond the plating/stripping reaction, and TFSI– can– withstand a high potential (>5.0 V vs. Na) [29]; Na plating/stripping reaction, and TFSI can withstand a high potential (>5.0 V vs. Na) moreover, excellent properties of ILs such as non-flammability, a low vapor pressure, wide [29]; moreover, excellent properties of ILs such as non-flammability, a low vapor pressure, electrochemical stability, and high thermal stability guarantee the safety of electrolyte wide electrochemical stability, and high thermal stability guarantee the safety of electro- materials [30,31]. A D-gluconic acetal-based gelator (B8, Chart 1) was selected to gel the lyte materials [30,31]. A D-gluconic acetal-based gelator (B8, Chart 1) was selected to gel IL [27]. Fortunately, the resultant supramolecular ionogel electrolyte based on B8, BMPTFSI, the IL [27]. Fortunately, the resultant supramolecular ionogel electrolyte based on B8, and NaTFSI (Chart 1) exhibited unprecedented performance, including favorable ionic BMPTFSI, and NaTFSI (Chart 1) exhibited unprecedented performance, including favor- conductivity, good Na+ transferen+ce number (T able ionic conductivity, good Na transference number (TNa ), high mechanical strength, +), high me+chanical strength, chemical stability, thixotropy, and a rapid self-healing property, indicative of a novel intelligent gel chemical stability, thixotropy, and a rapid self-healing property, indicative of a novel in- electrolyte with application potential for sodium batteries. Thixotropy, as well as stimuli- telligent gel electrolyte with application potential for sodium batteries. Thixotropy, as well responsive liquid-/solid-phase transitions, are significant for the molding of materials as stimuli-responsive liquid-/solid-phase transitions, are significant for the molding of in several industrial processes, including paints, ceramic sols, and device assembly [32]. materials in several industrial processes, including paints, ceramic sols, and device assem- Moreover, the self-assembly and thixotropic mechanism of the supramolecular ionogel bly [32]. Moreover, the self-assembly and thixotropic mechanism of the supramolecular electrolyte were investigated. Addressing the shortage of lithium resources and bright ionogel electrolyte were investigated. Addressing the shortage of lithium resources and prospect of NIBs, our studies on developing a multifunctional ionogel electrolyte provide bright prospect of NIBs, our studies on developing a multifunctional ionogel electrolyte some insights into the future development of high-performance supramolecular ionogel provide some insights into the future development of high-performance supramolecular electrolytes for NIBs. ionogel electrolytes for NIBs. Na Chart 1. Chemical structures of B8, BMPTFSI, and NaTFSI.PDF Image | Thixotropic Ionogel Electrolyte for Sodium Batteries
PDF Search Title:
Thixotropic Ionogel Electrolyte for Sodium BatteriesOriginal File Name Searched:
gels-08-00193-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |