
PDF Publication Title:
Text from PDF Page: 007
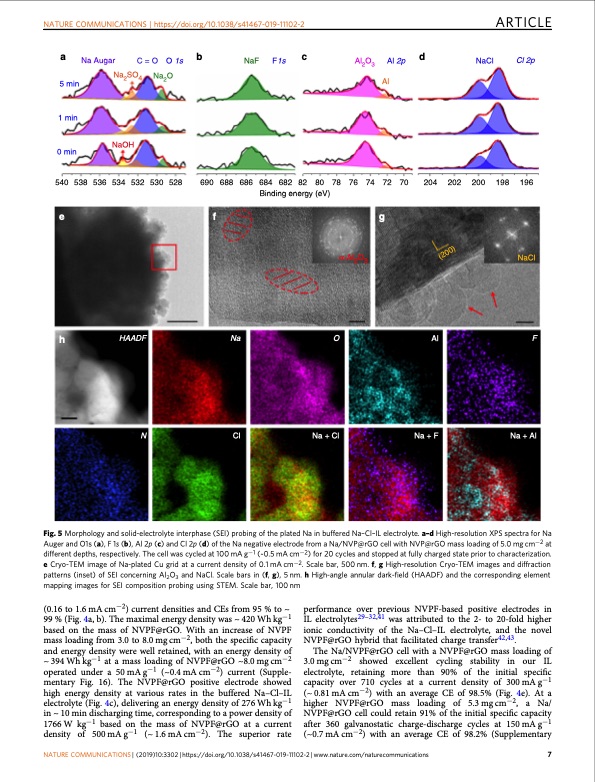
NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-019-11102-2 ARTICLE a Na Augar C = O O 1s b NaF F1s c Al2O3 Al 2p d Al NaCl Cl 2p 5 min 1 min 0 min Na2SO4 NaOH Na2O 540538536534532530528 69068868668468282 80 78 76 74 72 70 204 202 200 198 196 Binding energy (eV) efg h α-Al2O3 NaCl HAADF Na O Al F N Cl Na + Cl Na + F Na + Al Fig. 5 Morphology and solid-electrolyte interphase (SEI) probing of the plated Na in buffered Na–Cl–IL electrolyte. a–d High-resolution XPS spectra for Na Auger and O1s (a), F 1s (b), Al 2p (c) and Cl 2p (d) of the Na negative electrode from a Na/NVP@rGO cell with NVP@rGO mass loading of 5.0 mg cm−2 at different depths, respectively. The cell was cycled at 100 mA g−1 (~0.5 mA cm−2) for 20 cycles and stopped at fully charged state prior to characterization. e Cryo-TEM image of Na-plated Cu grid at a current density of 0.1 mA cm−2. Scale bar, 500 nm. f, g High-resolution Cryo-TEM images and diffraction patterns (inset) of SEI concerning Al2O3 and NaCl. Scale bars in (f, g), 5 nm. h High-angle annular dark-field (HAADF) and the corresponding element mapping images for SEI composition probing using STEM. Scale bar, 100 nm (0.16 to 1.6 mA cm−2) current densities and CEs from 95 % to ~ 99 % (Fig. 4a, b). The maximal energy density was ~ 420 Wh kg−1 based on the mass of NVPF@rGO. With an increase of NVPF mass loading from 3.0 to 8.0 mg cm−2, both the specific capacity and energy density were well retained, with an energy density of ~ 394 Wh kg−1 at a mass loading of NVPF@rGO ~8.0 mg cm−2 operated under a 50 mA g−1 (~0.4 mA cm−2) current (Supple- mentary Fig. 16). The NVPF@rGO positive electrode showed high energy density at various rates in the buffered Na–Cl–IL electrolyte (Fig. 4c), delivering an energy density of 276 Wh kg−1 in ~ 10 min discharging time, corresponding to a power density of 1766 W kg−1 based on the mass of NVPF@rGO at a current density of 500 mA g−1 (~ 1.6 mA cm−2). The superior rate performance over previous NVPF-based positive electrodes in IL electrolytes29–32,41 was attributed to the 2- to 20-fold higher ionic conductivity of the Na–Cl–IL electrolyte, and the novel NVPF@rGO hybrid that facilitated charge transfer42,43. The Na/NVPF@rGO cell with a NVPF@rGO mass loading of 3.0 mg cm−2 showed excellent cycling stability in our IL electrolyte, retaining more than 90% of the initial specific capacity over 710 cycles at a current density of 300 mA g−1 (~ 0.81 mA cm−2) with an average CE of 98.5% (Fig. 4e). At a higher NVPF@rGO mass loading of 5.3 mg cm−2, a Na/ NVPF@rGO cell could retain 91% of the initial specific capacity after 360 galvanostatic charge-discharge cycles at 150 mA g−1 (~0.7 mA cm−2) with an average CE of 98.2% (Supplementary NATURE COMMUNICATIONS | (2019)10:3302 | https://doi.org/10.1038/s41467-019-11102-2 | www.nature.com/naturecommunications 7 (200)PDF Image | sodium metal battery based on an ionic liquid electrolyte
PDF Search Title:
sodium metal battery based on an ionic liquid electrolyteOriginal File Name Searched:
s41467-019-11102-2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |