
PDF Publication Title:
Text from PDF Page: 006
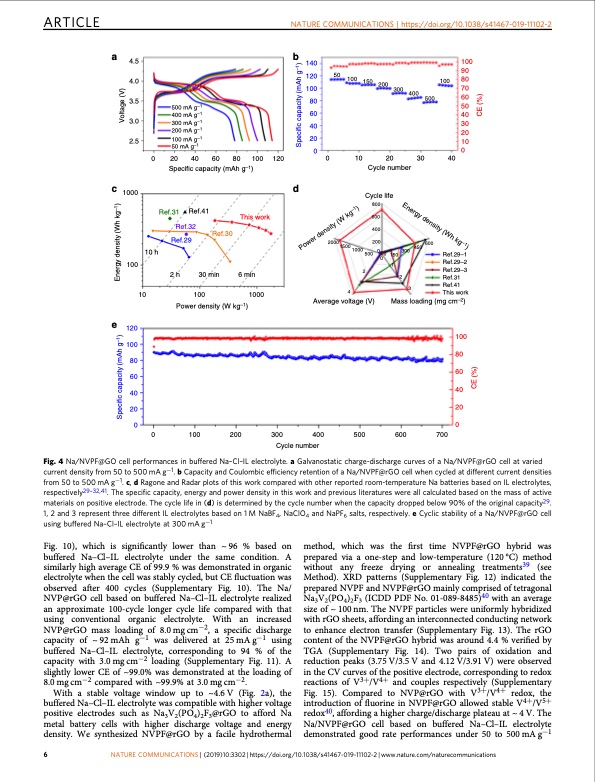
ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-019-11102-2 a 4.5 4.0 3.5 3.0 2.5 c 1000 100 e 120 100 80 60 40 20 b 140 120 100 80 60 40 20 50 100 150 200 300 100 90 100 80 70 60 50 40 30 20 10 500 mA g–1 400 mA g–1 300 mA g–1 200 mA g–1 100 mA g–1 50 mA g–1 0 20 40 60 80 100 120 Specific capacity (mAh g–1) 00 d Cycle life 800 0 10 20 30 Cycle number 40 Ref.29–1 Ref.29–2 Ref.29–3 Ref.31 Ref.41 This work 400 500 10 h 10 500 0 0 150 1 300 450 2 Ref.31 Ref.41 Ref.32 Ref.29 2 h This work Ref.30 30 min 2000 15001000 2 600 400 200 600 100 Power density (W kg–1) 6 min 1000 4 Average voltage (V) 3 Mass loading (mg cm–2) 0 00 0 100 200 300 400 500 600 700 Cycle number Fig. 4 Na/NVPF@GO cell performances in buffered Na–Cl–IL electrolyte. a Galvanostatic charge-discharge curves of a Na/NVPF@rGO cell at varied current density from 50 to 500 mA g−1. b Capacity and Coulombic efficiency retention of a Na/NVPF@rGO cell when cycled at different current densities from 50 to 500 mA g−1. c, d Ragone and Radar plots of this work compared with other reported room-temperature Na batteries based on IL electrolytes, respectively29–32,41. The specific capacity, energy and power density in this work and previous literatures were all calculated based on the mass of active materials on positive electrode. The cycle life in (d) is determined by the cycle number when the capacity dropped below 90% of the original capacity29. 1, 2 and 3 represent three different IL electrolytes based on 1 M NaBF4, NaClO4 and NaPF6 salts, respectively. e Cyclic stability of a Na/NVPF@rGO cell using buffered Na–Cl–IL electrolyte at 300 mA g−1 Fig. 10), which is significantly lower than ~96 % based on buffered Na–Cl–IL electrolyte under the same condition. A similarly high average CE of 99.9 % was demonstrated in organic electrolyte when the cell was stably cycled, but CE fluctuation was observed after 400 cycles (Supplementary Fig. 10). The Na/ NVP@rGO cell based on buffered Na–Cl–IL electrolyte realized an approximate 100-cycle longer cycle life compared with that using conventional organic electrolyte. With an increased NVP@rGO mass loading of 8.0 mg cm−2, a specific discharge capacity of ~ 92 mAh g−1 was delivered at 25 mA g−1 using buffered Na–Cl–IL electrolyte, corresponding to 94 % of the capacity with 3.0 mg cm−2 loading (Supplementary Fig. 11). A slightly lower CE of ~99.0% was demonstrated at the loading of 8.0 mg cm−2 compared with ~99.9% at 3.0 mg cm−2. With a stable voltage window up to ~4.6V (Fig. 2a), the buffered Na–Cl–IL electrolyte was compatible with higher voltage positive electrodes such as Na3V2(PO4)2F3@rGO to afford Na metal battery cells with higher discharge voltage and energy density. We synthesized NVPF@rGO by a facile hydrothermal method, which was the first time NVPF@rGO hybrid was prepared via a one-step and low-temperature (120 °C) method without any freeze drying or annealing treatments39 (see Method). XRD patterns (Supplementary Fig. 12) indicated the prepared NVPF and NVPF@rGO mainly comprised of tetragonal Na3V2(PO4)2F3 (ICDD PDF No. 01-089-8485)40 with an average size of ~ 100 nm. The NVPF particles were uniformly hybridized with rGO sheets, affording an interconnected conducting network to enhance electron transfer (Supplementary Fig. 13). The rGO content of the NVPF@rGO hybrid was around 4.4 % verified by TGA (Supplementary Fig. 14). Two pairs of oxidation and reduction peaks (3.75 V/3.5 V and 4.12 V/3.91 V) were observed in the CV curves of the positive electrode, corresponding to redox reactions of V3+/V4+ and couples respectively (Supplementary Fig. 15). Compared to NVP@rGO with V3+/V4+ redox, the introduction of fluorine in NVPF@rGO allowed stable V4+/V5+ redox40, affording a higher charge/discharge plateau at ~ 4 V. The Na/NVPF@rGO cell based on buffered Na–Cl–IL electrolyte demonstrated good rate performances under 50 to 500 mA g−1 6 NATURE COMMUNICATIONS | (2019)10:3302 | https://doi.org/10.1038/s41467-019-11102-2 | www.nature.com/naturecommunications 100 80 60 40 20 Energy density (Wh kg–1) Power density (W kg–1) Specific capacity (mAh g–1) Energy density (Wh kg–1) Voltage (V) CE (%) CE (%) Specific capacity (mAh g–1)PDF Image | sodium metal battery based on an ionic liquid electrolyte
PDF Search Title:
sodium metal battery based on an ionic liquid electrolyteOriginal File Name Searched:
s41467-019-11102-2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |