
PDF Publication Title:
Text from PDF Page: 004
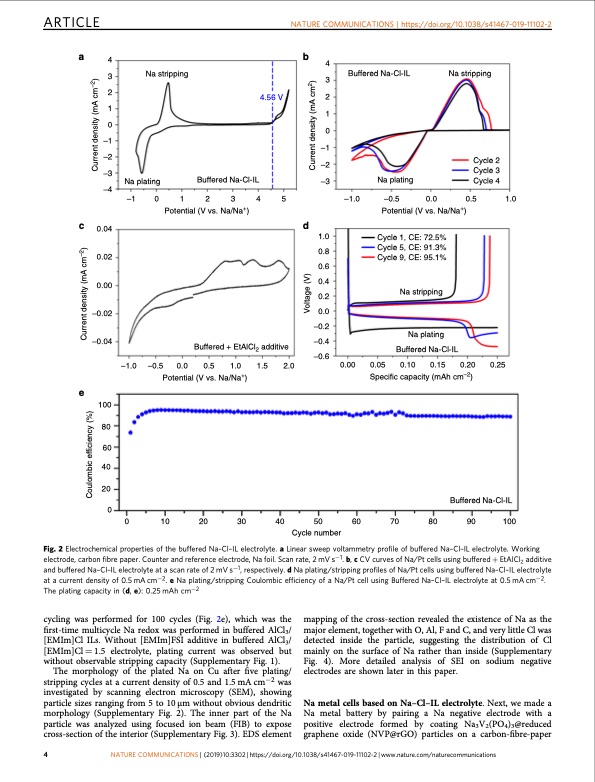
ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-019-11102-2 a4b 4 3 2 1 0 –1 –2 –3 –4 c 0.04 0.02 0.00 –0.02 –0.04 Na stripping Na plating 3 4.56 V 2 1 0 –1 –2 Buffered Na-Cl-IL –3 Buffered Na-Cl-IL Na plating Na stripping Cycle 2 Cycle 3 Cycle 4 –1 0 1 2 3 4 5 Potential (V vs. Na/Na+) –1.0 –0.5 0.0 0.5 1.0 Potential (V vs. Na/Na+) d e 100 80 60 40 20 0 Buffered Na-Cl-IL –1.0 –0.5 0.0 Potential (V vs. Na/Na+) 0.00 Buffered + EtAlCl 2 additive –0.4 –0.6 0.5 1.0 1.5 2.0 1.0 0.8 0.6 0.4 0.2 0.0 –0.2 Cycle 1, CE: 72.5% Cycle 5, CE: 91.3% Cycle 9, CE: 95.1% Na stripping Na plating Buffered Na-Cl-IL 0.05 0.10 0.15 0.20 0.25 Specific capacity (mAh cm–2) 0 10 20 30 40 Cycle 50 60 70 80 90 100 number Fig. 2 Electrochemical properties of the buffered Na–Cl–IL electrolyte. a Linear sweep voltammetry profile of buffered Na–Cl–IL electrolyte. Working electrode, carbon fibre paper. Counter and reference electrode, Na foil. Scan rate, 2 mV s−1. b, c CV curves of Na/Pt cells using buffered + EtAlCl2 additive and buffered Na–Cl–IL electrolyte at a scan rate of 2 mV s−1, respectively. d Na plating/stripping profiles of Na/Pt cells using buffered Na–Cl–IL electrolyte at a current density of 0.5 mA cm−2. e Na plating/stripping Coulombic efficiency of a Na/Pt cell using Buffered Na–Cl–IL electrolyte at 0.5 mA cm−2. The plating capacity in (d, e): 0.25 mAh cm−2 cycling was performed for 100 cycles (Fig. 2e), which was the first-time multicycle Na redox was performed in buffered AlCl3/ [EMIm]Cl ILs. Without [EMIm]FSI additive in buffered AlCl3/ [EMIm]Cl = 1.5 electrolyte, plating current was observed but without observable stripping capacity (Supplementary Fig. 1). The morphology of the plated Na on Cu after five plating/ stripping cycles at a current density of 0.5 and 1.5 mA cm−2 was investigated by scanning electron microscopy (SEM), showing particle sizes ranging from 5 to 10 μm without obvious dendritic morphology (Supplementary Fig. 2). The inner part of the Na particle was analyzed using focused ion beam (FIB) to expose cross-section of the interior (Supplementary Fig. 3). EDS element mapping of the cross-section revealed the existence of Na as the major element, together with O, Al, F and C, and very little Cl was detected inside the particle, suggesting the distribution of Cl mainly on the surface of Na rather than inside (Supplementary Fig. 4). More detailed analysis of SEI on sodium negative electrodes are shown later in this paper. Na metal cells based on Na–Cl–IL electrolyte. Next, we made a Na metal battery by pairing a Na negative electrode with a positive electrode formed by coating Na3V2(PO4)3@reduced graphene oxide (NVP@rGO) particles on a carbon-fibre-paper 4 NATURE COMMUNICATIONS | (2019)10:3302 | https://doi.org/10.1038/s41467-019-11102-2 | www.nature.com/naturecommunications Coulombic efficiency (%) Current density (mA cm–2) Voltage (V) Current density (mA cm2) Current density (mA cm–2)PDF Image | sodium metal battery based on an ionic liquid electrolyte
PDF Search Title:
sodium metal battery based on an ionic liquid electrolyteOriginal File Name Searched:
s41467-019-11102-2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |