
PDF Publication Title:
Text from PDF Page: 003
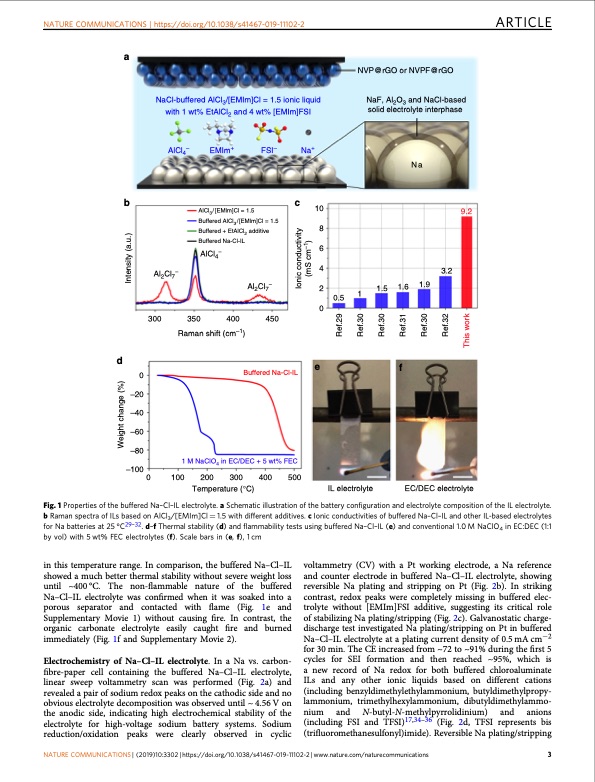
NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-019-11102-2 ARTICLE a b EMIm+ FSI– AlCl3/[EMlm]Cl = 1.5 Buffered AlCl3/[EMlm]Cl = 1.5 Buffered + EtAlCl2 additive Buffered Na-Cl-IL AlCl4− Al2Cl7− 350 400 450 Raman shift (cm–1) Buffered Na-Cl-IL 1 M NaClO4 in EC/DEC + 5 wt% FEC 100 200 300 400 500 Temperature (°C) Na 3.2 1.5 1.6 1.9 NaCl-buffered AlCl3/[EMIm]Cl = 1.5 ionic liquid with 1 wt% EtAlCl2 and 4 wt% [EMIm]FSI NaF, Al2O3 and NaCl-based solid electrolyte interphase AlCl4– Al2Cl7− Na+ c 10 8 6 4 2 0 NVP@rGO or NVPF@rGO d 0 –20 –40 –60 –80 –100 300 1 0.5 ef IL electrolyte 0 Fig. 1 Properties of the buffered Na–Cl–IL electrolyte. a Schematic illustration of the battery configuration and electrolyte composition of the IL electrolyte. b Raman spectra of ILs based on AlCl3/[EMIm]Cl = 1.5 with different additives. c Ionic conductivities of buffered Na–Cl–IL and other IL-based electrolytes for Na batteries at 25 °C29–32. d–f Thermal stability (d) and flammability tests using buffered Na–Cl–IL (e) and conventional 1.0 M NaClO4 in EC:DEC (1:1 by vol) with 5 wt% FEC electrolytes (f). Scale bars in (e, f), 1 cm in this temperature range. In comparison, the buffered Na–Cl–IL showed a much better thermal stability without severe weight loss until ~400°C. The non-flammable nature of the buffered Na–Cl–IL electrolyte was confirmed when it was soaked into a porous separator and contacted with flame (Fig. 1e and Supplementary Movie 1) without causing fire. In contrast, the organic carbonate electrolyte easily caught fire and burned immediately (Fig. 1f and Supplementary Movie 2). Electrochemistry of Na–Cl–IL electrolyte. In a Na vs. carbon- fibre-paper cell containing the buffered Na–Cl–IL electrolyte, linear sweep voltammetry scan was performed (Fig. 2a) and revealed a pair of sodium redox peaks on the cathodic side and no obvious electrolyte decomposition was observed until ~ 4.56 V on the anodic side, indicating high electrochemical stability of the electrolyte for high-voltage sodium battery systems. Sodium reduction/oxidation peaks were clearly observed in cyclic voltammetry (CV) with a Pt working electrode, a Na reference and counter electrode in buffered Na–Cl–IL electrolyte, showing reversible Na plating and stripping on Pt (Fig. 2b). In striking contrast, redox peaks were completely missing in buffered elec- trolyte without [EMIm]FSI additive, suggesting its critical role of stabilizing Na plating/stripping (Fig. 2c). Galvanostatic charge- discharge test investigated Na plating/stripping on Pt in buffered Na–Cl–IL electrolyte at a plating current density of 0.5 mA cm−2 for 30 min. The CE increased from ~72 to ~91% during the first 5 cycles for SEI formation and then reached ~95%, which is a new record of Na redox for both buffered chloroaluminate ILs and any other ionic liquids based on different cations (including benzyldimethylethylammonium, butyldimethylpropy- lammonium, trimethylhexylammonium, dibutyldimethylammo- nium and N-butyl-N-methylpyrrolidinium) and anions (including FSI and TFSI)17,34–36 (Fig. 2d, TFSI represents bis (trifluoromethanesulfonyl)imide). Reversible Na plating/stripping NATURE COMMUNICATIONS | (2019)10:3302 | https://doi.org/10.1038/s41467-019-11102-2 | www.nature.com/naturecommunications 3 EC/DEC electrolyte 9.2 Weight change (%) Ref.29 Ref.30 Ref.30 Ref.31 Ref.30 Ref.32 This work Intensity (a.u.) Ionic conductivity (mS cm–1)PDF Image | sodium metal battery based on an ionic liquid electrolyte
PDF Search Title:
sodium metal battery based on an ionic liquid electrolyteOriginal File Name Searched:
s41467-019-11102-2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |