
PDF Publication Title:
Text from PDF Page: 060
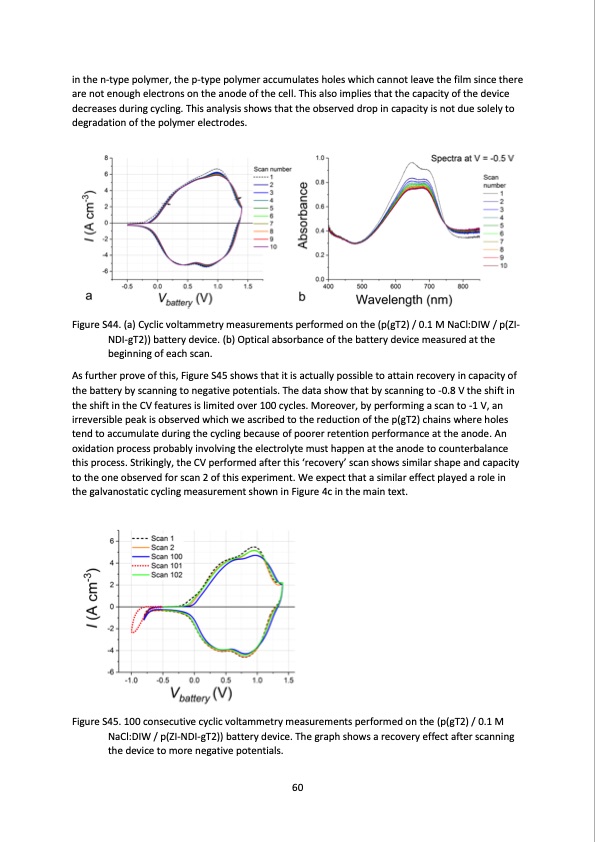
in the n-type polymer, the p-type polymer accumulates holes which cannot leave the film since there are not enough electrons on the anode of the cell. This also implies that the capacity of the device decreases during cycling. This analysis shows that the observed drop in capacity is not due solely to degradation of the polymer electrodes. Figure S44. (a) Cyclic voltammetry measurements performed on the (p(gT2) / 0.1 M NaCl:DIW / p(ZI- NDI-gT2)) battery device. (b) Optical absorbance of the battery device measured at the beginning of each scan. As further prove of this, Figure S45 shows that it is actually possible to attain recovery in capacity of the battery by scanning to negative potentials. The data show that by scanning to -0.8 V the shift in the shift in the CV features is limited over 100 cycles. Moreover, by performing a scan to -1 V, an irreversible peak is observed which we ascribed to the reduction of the p(gT2) chains where holes tend to accumulate during the cycling because of poorer retention performance at the anode. An oxidation process probably involving the electrolyte must happen at the anode to counterbalance this process. Strikingly, the CV performed after this ‘recovery’ scan shows similar shape and capacity to the one observed for scan 2 of this experiment. We expect that a similar effect played a role in the galvanostatic cycling measurement shown in Figure 4c in the main text. Figure S45. 100 consecutive cyclic voltammetry measurements performed on the (p(gT2) / 0.1 M NaCl:DIW / p(ZI-NDI-gT2)) battery device. The graph shows a recovery effect after scanning the device to more negative potentials. 60PDF Image | salt water battery with high stability
PDF Search Title:
salt water battery with high stabilityOriginal File Name Searched:
salt-water-battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |