
PDF Publication Title:
Text from PDF Page: 059
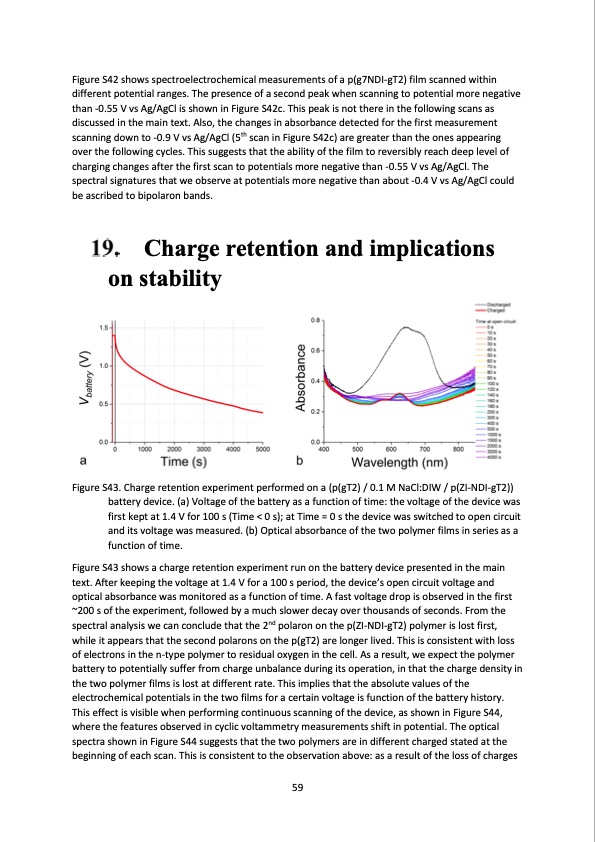
Figure S42 shows spectroelectrochemical measurements of a p(g7NDI-gT2) film scanned within different potential ranges. The presence of a second peak when scanning to potential more negative than -0.55 V vs Ag/AgCl is shown in Figure S42c. This peak is not there in the following scans as discussed in the main text. Also, the changes in absorbance detected for the first measurement scanning down to -0.9 V vs Ag/AgCl (5th scan in Figure S42c) are greater than the ones appearing over the following cycles. This suggests that the ability of the film to reversibly reach deep level of charging changes after the first scan to potentials more negative than -0.55 V vs Ag/AgCl. The spectral signatures that we observe at potentials more negative than about -0.4 V vs Ag/AgCl could be ascribed to bipolaron bands. Charge retention and implications on stability Figure S43. Charge retention experiment performed on a (p(gT2) / 0.1 M NaCl:DIW / p(ZI-NDI-gT2)) battery device. (a) Voltage of the battery as a function of time: the voltage of the device was first kept at 1.4 V for 100 s (Time < 0 s); at Time = 0 s the device was switched to open circuit and its voltage was measured. (b) Optical absorbance of the two polymer films in series as a function of time. Figure S43 shows a charge retention experiment run on the battery device presented in the main text. After keeping the voltage at 1.4 V for a 100 s period, the device’s open circuit voltage and optical absorbance was monitored as a function of time. A fast voltage drop is observed in the first ~200 s of the experiment, followed by a much slower decay over thousands of seconds. From the spectral analysis we can conclude that the 2nd polaron on the p(ZI-NDI-gT2) polymer is lost first, while it appears that the second polarons on the p(gT2) are longer lived. This is consistent with loss of electrons in the n-type polymer to residual oxygen in the cell. As a result, we expect the polymer battery to potentially suffer from charge unbalance during its operation, in that the charge density in the two polymer films is lost at different rate. This implies that the absolute values of the electrochemical potentials in the two films for a certain voltage is function of the battery history. This effect is visible when performing continuous scanning of the device, as shown in Figure S44, where the features observed in cyclic voltammetry measurements shift in potential. The optical spectra shown in Figure S44 suggests that the two polymers are in different charged stated at the beginning of each scan. This is consistent to the observation above: as a result of the loss of charges 59PDF Image | salt water battery with high stability
PDF Search Title:
salt water battery with high stabilityOriginal File Name Searched:
salt-water-battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |