
PDF Publication Title:
Text from PDF Page: 005
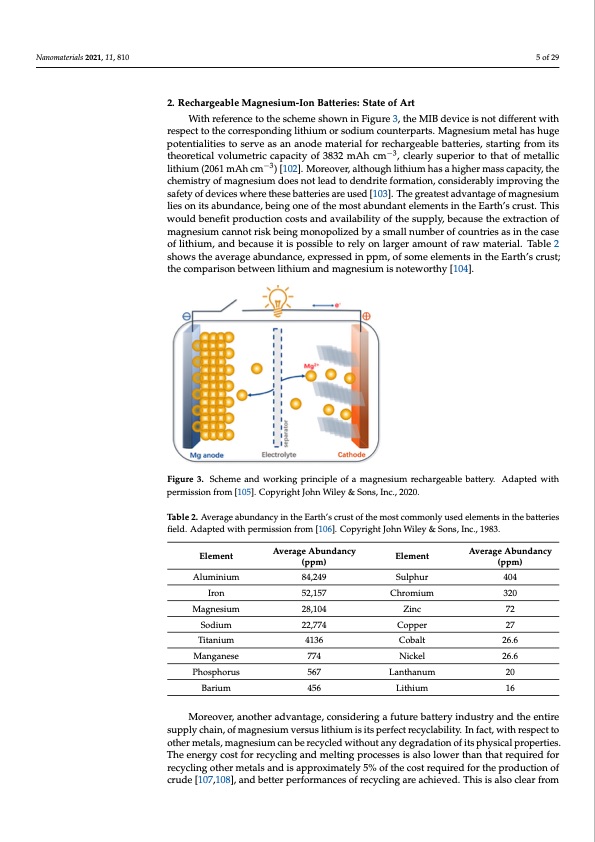
Nanomaterials 2021, 11, 810 Nanomaterials 2021, 11, x 5 of 29 5 of 28 2. Rechargeable Magnesium-Ion Batteries: State of Art With reference to the scheme shown in Figure 3, the MIB device is not different with With reference to the scheme shown in Figure 3, the MIB device is not different with respect to the corresponding lithium or sodium counterparts. Magnesium metal has huge respect to the corresponding lithium or sodium counterparts. Magnesium metal has huge potentialities to serve as an anode material for rechargeable batteries, starting from its potentialities to serve as an anode material for rechargeable batteries, starting from its −3 theoretical volumetric capacity of 3832 mAh cm −, 3clearly superior to that of metallic lith- theoretical volumetric capacity of 3832 mAh cm , clearly superior to that of metallic ium (2061 mAh cm−3)−[3102]. Moreover, although lithium has a higher mass capacity, the lithium (2061 mAh cm ) [102]. Moreover, although lithium has a higher mass capacity, the chemistry of magnesium does not lead to dendrite formation, considerably improving the chemistry of magnesium does not lead to dendrite formation, considerably improving the safety of devices where these batteries are used [103]. The greatest advantage of magne- safety of devices where these batteries are used [103]. The greatest advantage of magnesium sium lies on its abundance, being one of the most abundant elements in the Earth’s crust. lies on its abundance, being one of the most abundant elements in the Earth’s crust. This This would benefit production costs and availability of the supply, because the extraction would benefit production costs and availability of the supply, because the extraction of of magnesium cannot risk being monopolized by a small number of countries as in the magnesium cannot risk being monopolized by a small number of countries as in the case case of lithium, and because it is possible to rely on larger amount of raw material. Table of lithium, and because it is possible to rely on larger amount of raw material. Table 2 2 shows the average abundance, expressed in ppm, of some elements in the Earth’s crust; shows the average abundance, expressed in ppm, of some elements in the Earth’s crust; the comparison between lithium and magnesium is noteworthy [104]. the comparison between lithium and magnesium is noteworthy [104]. Figure 3. Scheme and working principle of a magnesium rechargeable battery. Adapted with per- Figure 3. Scheme and working principle of a magnesium rechargeable battery. Adapted with teries field. Adapted with permission from [106]. Copyright John Wiley & Sons, Inc., 1983. mission from [105]. Copyright John Wiley & Sons, Inc., 2020. permission from [105]. Copyright John Wiley & Sons, Inc., 2020. Table 2. Average abundancy in the Earth’s crust of the most commonly used elements in the bat- Table 2. Average abundancy in the Earth’s crust of the most commonly used elements in the batteries field. Adapted with permission from [106]. Copyright John Wiley & Sons, Inc., 1983. Element AlumEilneimument Iron Average Abundancy (ppm) Element SulEplheumrent Chromium Average Abundancy (ppm) Average Abundancy Average Abundancy 84,249 404 (ppm) (ppm) 52,157 320 Aluminium 84,249 404 CCophproemrium 27320 72 27 20 16 26.6 Magnesium SodiuIrmon 28,104 22,75724,157 Zinc Cobalt 26.6 Sulphur 72 Titanium 4136 Magnesium 28,104 Zinc Manganese 774 Nickel 26.6 Sodium 22,774 Copper Phosphorus 567 Lanthanum 26.6 Titanium 4136 Cobalt Barium 456 Lithium Manganese 774 Nickel Moreover, another advantage, considering a future battery industry and the entire Phosphorus 567 Lanthanum 20 supply chain, of magnesium versus lithium is its perfect recyclability. In fact, with respect Barium 456 Lithium 16 to other metals, magnesium can be recycled without any degradation of its physical prop- erties. The energy cost for recycling and melting processes is also lower than that required Moreover, another advantage, considering a future battery industry and the entire for recycling other metals and is approximately 5% of the cost required for the production supply chain, of magnesium versus lithium is its perfect recyclability. In fact, with respect to of crude [107,108], and better performances of recycling are achieved. This is also clear other metals, magnesium can be recycled without any degradation of its physical properties. from Figure 4, that shows the end-of-life recycling rate of some elements of the periodic The energy cost for recycling and melting processes is also lower than that required for table, i.e., the ratio between the amount of element truly recycled and the total quantity of recycling other metals and is approximately 5% of the cost required for the production of element introduced in the recycling flow (noteworthy comparing the 25–50% of magne- crude [107,108], and better performances of recycling are achieved. This is also clear from sium vs. less than 1% for lithium).PDF Image | Overview on Anodes for Magnesium Batteries
PDF Search Title:
Overview on Anodes for Magnesium BatteriesOriginal File Name Searched:
nanomaterials-11-00810.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |