
PDF Publication Title:
Text from PDF Page: 006
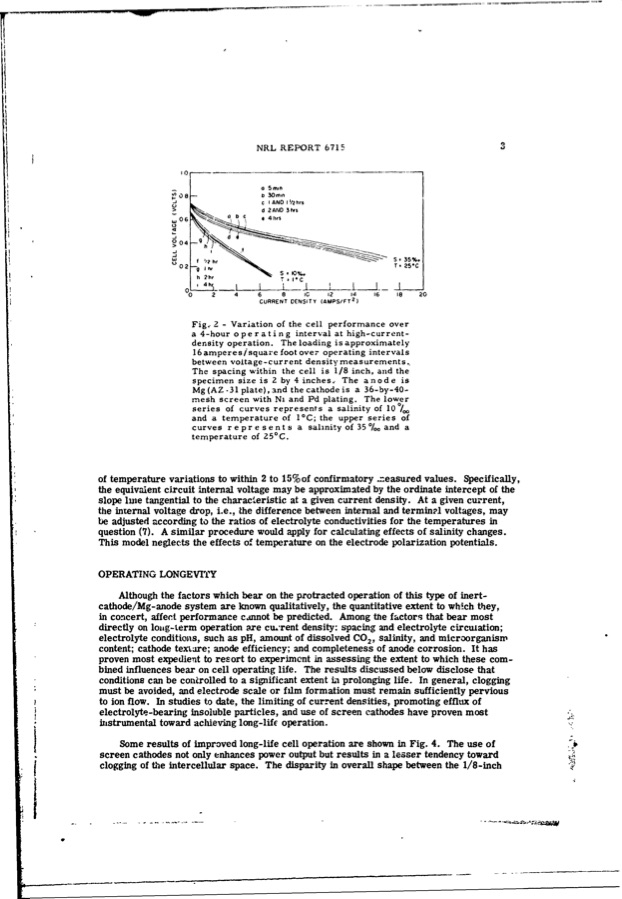
U I of temperature variations to within 2 to 15%of confirmatory .neasured values. Specifically, the equivalent circuit internal voltage may be approximated by the ordinate intercept of the slope line tangential to the characteristic at a given current density. At a given current, the internal voltage drop, i.e., the difference between internal and terminll voltages, may be adjusted according to the ratios of electrolyte conductivities for the temperatures in question (7). A similar procedure would apply for calculating effects of salinity changes. This model neglects the effects of temperature on the electrode polarization potentials. OPERATING LONGEVITY Although the factors which bear on the protracted operation of this type of inert- cathode/Mg-anode system are known qualitatively, the quantitative extent to which they, in concert, affect performance cannot be predicted. Among the factors that bear most directly on loug-term operation axe current density: spacing and electrolyte circulation; electrolyte conditions, such as pH, amount of dissolved CO2 salinity, and microorganism content; cathode texmare; anode efficiency; and completeness of anode corrosion. It has proven most expedient to resort to experiment in assessing the extent to which these com- bined influences bear on cell operating life. The results discussed below disclose that conditions can be controlled to a significant extent La prolonging life. In general, clogging must be avoided, and electrode scale or film formation must remain sufficiently pervious to ion flow. In studies to date, the limiting of current densities, promoting efflux of electrolyte-bearing insoluble particles, and use of screen cathodes have proven most instrumental toward achieving long-life operation. Some results of improved long-life cell operation are shown in Fig. 4. The use of screen cathodes not only enhances power output but results in a lesser tendency toward clogging of the intercellular space. The disparity in overall shape between the 1/8-inch Isae •0 8 W06 0 04 a 5mm D3Omr C I ANDI'/2hrs d 2ANJD3tws 4rs u f '?2hr h 2hr S , 35%. T 25"C 0T2 - NRL REPORT 6715 3 " - I*C -I-- 0 2 4 6 8 IC 12 124 16 18 20 L-_____L_____LL_ CURRENT DENSITY (AMPS/FT ) Fig, 2 - Variation of the cell performance over a 4-hour operating interval at high-current- density operation. The loading is approximately 16 amperes/square foot over operating intervals between voltage-current density measurements., The spacing within the cell is 1/8 inch, and the specimen size is 2 by 4 inches, The anode is Mg (AZ -31 plate), and the cathode is a 36-by-40- mesh screen with Ni and Pd plating. The lower series of curves represents a salinity of 10 0/. and a temperature of I°C; the upper series of curves represents a sahnity of 350%/ and a temperature of 25°C. __PDF Image | INERT-CATHODE SEA-WATER BATTERY
PDF Search Title:
INERT-CATHODE SEA-WATER BATTERYOriginal File Name Searched:
AD0673399.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |