
PDF Publication Title:
Text from PDF Page: 005
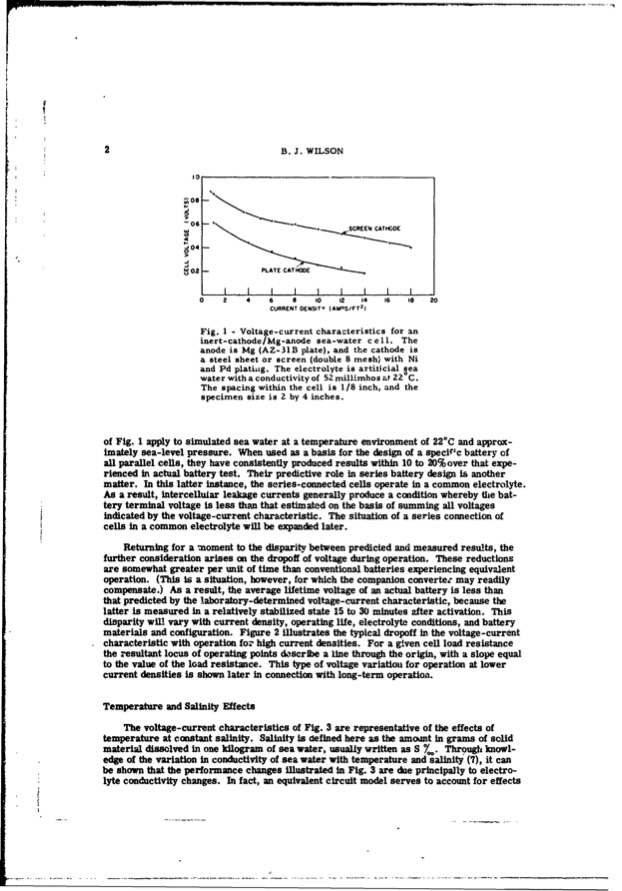
2 B. J. WILSON S-. • _.e..SCREEN C.AHTODE PLATE CATHODE I,IIIIIIII t~O I- 804 > -02 0 2 4 6 8 10 12 14 16 Is 20 2 CURRENT DENSITV (AMOS/FT ) Fig. I - Voltage-current characteristics for an inert-cathode/ Mg-anode sea-water c eli. The anode is Mg (AZ-31B plate), and the cathode is a steel sheet or screen (double 8 mesh) with Ni and Pd platihig. The electrolyte is artiticial sea 0 water with a conductivity of 52Zmillimhos at Z2 C. The spacing within the cell is 1/8 inch, and the specimen size is 2 by 4 inches. of Fig. I apply to simulated sea water at a temperature environment of 22*C and approx- imately sea-level pressure. When used as a basis for the design of a specil'c battery of all parallel cells, they have consistently produced results within 10 to 20% over that expe- rienced in actual battery test. Their predictive role in series battery design is another matter. In this latter instance, the series-connected cells operate in a common electrolyte. As a result, intercellular leakage currents generally produce a condition whereby the bat- tery terminal voltage is less than that estimated on the basis of summing all voltages indicated by the voltage-current characteristic. The situation of a series connection of cells in a common electrolyte will be expanded later. Returning for a moment to the disparity between predicted and measured results, the further consideration arises on the dropoff of voltage during operation. These reductions are somewhat greater per unit of time than conventional batteries experiencing equivalent operation. (This is a situation, however, for which the companion converter may readily compensate.) As a result, the average lifetime voltage of an actual battery is less than that predicted by the laboratory-determined voltage-current characteristic, because the latter is measured in a relatively stabilized state 15 to 30 minutes after activation. This disparity will vary with current density, operating life, electrolyte conditions, and battery materials and configuration. Figure 2 illustrates the typical dropoff in the voltage-current characteristic with operation for high current densities. For a given cell load resistance the resultant locus of operating points de'scribe a line through the origin, with a slope equal to the value of the load resistance. This type of voltage variation for operation at lower current densities is shown later in connection with long-term operation. Temperature and Salinity Effects The voltage-current characteristics of Fig. 3 are representative of the effects of temperature at constant salinity. Salinity is defined here as the amonnt in grams of solid material dissolved in one kilogram of sea water, usually written as S 7,,. Through knowl- edge of the variation in conductivity of seawater with temperature and salinity (7), it can be shown that the performance changes illustrated in Fig. 3 are due principally to electro- lyte conductivity changes. In fact, an equivalent circuit model serves to account for effectsPDF Image | INERT-CATHODE SEA-WATER BATTERY
PDF Search Title:
INERT-CATHODE SEA-WATER BATTERYOriginal File Name Searched:
AD0673399.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |