
PDF Publication Title:
Text from PDF Page: 003
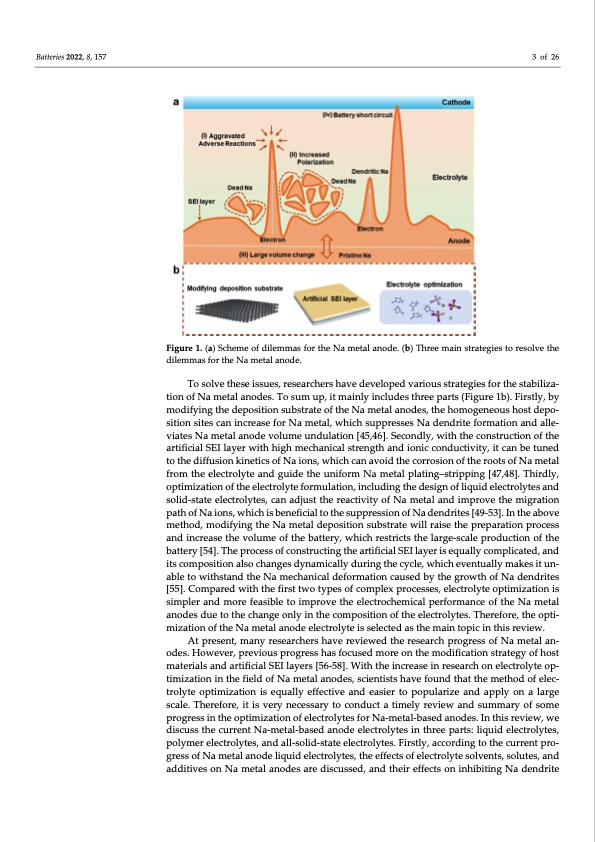
Batteries 2022, 8, 157 3 of 26 Figure 1. (a) Scheme of dilemmas for the Na metal anode. (b) Three main strategies to resolve the dilemmas for the Na metal anode. To solve these issues, researchers have developed various strategies for the stabiliza‐ tion of Na metal anodes. To sum up, it mainly includes three parts (Figure 1b). Firstly, by modifying the deposition substrate of the Na metal anodes, the homogeneous host depo‐ sition sites can increase for Na metal, which suppresses Na dendrite formation and alle‐ viates Na metal anode volume undulation [45,46]. Secondly, with the construction of the artificial SEI layer with high mechanical strength and ionic conductivity, it can be tuned to the diffusion kinetics of Na ions, which can avoid the corrosion of the roots of Na metal from the electrolyte and guide the uniform Na metal plating–stripping [47,48]. Thirdly, optimization of the electrolyte formulation, including the design of liquid electrolytes and solid‐state electrolytes, can adjust the reactivity of Na metal and improve the migration path of Na ions, which is beneficial to the suppression of Na dendrites [49‐53]. In the above method, modifying the Na metal deposition substrate will raise the preparation process and increase the volume of the battery, which restricts the large‐scale production of the battery [54]. The process of constructing the artificial SEI layer is equally complicated, and its composition also changes dynamically during the cycle, which eventually makes it un‐ able to withstand the Na mechanical deformation caused by the growth of Na dendrites [55]. Compared with the first two types of complex processes, electrolyte optimization is simpler and more feasible to improve the electrochemical performance of the Na metal anodes due to the change only in the composition of the electrolytes. Therefore, the opti‐ mization of the Na metal anode electrolyte is selected as the main topic in this review. At present, many researchers have reviewed the research progress of Na metal an‐ odes. However, previous progress has focused more on the modification strategy of host materials and artificial SEI layers [56‐58]. With the increase in research on electrolyte op‐ timization in the field of Na metal anodes, scientists have found that the method of elec‐ trolyte optimization is equally effective and easier to popularize and apply on a large scale. Therefore, it is very necessary to conduct a timely review and summary of some progress in the optimization of electrolytes for Na‐metal‐based anodes. In this review, we discuss the current Na‐metal‐based anode electrolytes in three parts: liquid electrolytes, polymer electrolytes, and all‐solid‐state electrolytes. Firstly, according to the current pro‐ gress of Na metal anode liquid electrolytes, the effects of electrolyte solvents, solutes, and additives on Na metal anodes are discussed, and their effects on inhibiting Na dendritePDF Image | Electrolyte Engineering for Sodium Metal Batteries
PDF Search Title:
Electrolyte Engineering for Sodium Metal BatteriesOriginal File Name Searched:
batteries-08-00157.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |