
PDF Publication Title:
Text from PDF Page: 008
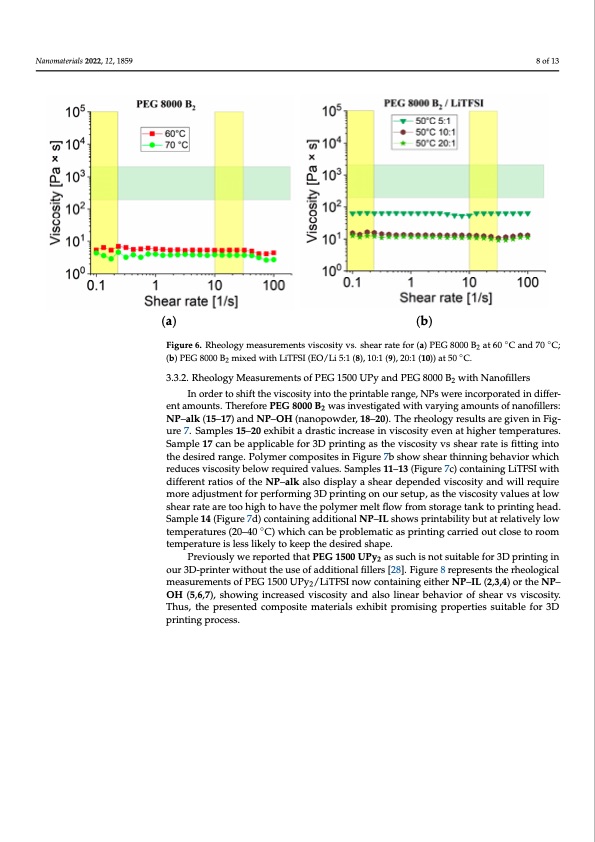
melt viscosity is far too low from the printing window (presented as cross section of green and yellow cross section), hence the sample is not applicable for 3D printing processes using fused deposition modeling. In addition, compositions containing PEG 8000 B2 mixed with different ratios of LiTFSI (8, 9 and 10) without nanofillers showed a lower Nanomaterials 2022, 12, 1859 8 of 13 viscosity than desired values at 50 °C given in Figure 6b. (a) (b) Figure6.RheologymeasFuigruerme6e.nRthsevoliosgcyomsietaysuvrse.msehnetsavrisrcaotseityfovrs.(sah)eaPrEraGte8fo0r0(0a)BP2EaGt8600°CB ant6d07C0a°nCd;7(0b)C; ◦ (b) PEG 8000 B mixed with LiTFSI (EO/Li 5:1 (8), 10:1 (9), 20:1 (10)) at 50 PEG 8000 B2 mixed with LiTFSI (EO2/Li 5:1 (8), 10:1 (9), 20:1 (10)) at 50 °C;. C. 3.3.2. Rheology Measurements of PEG 1500 UPy and PEG 8000 B2 with Nanofillers 3.3.2. Rheology Measurements of PEG 1500 UPy and PEG 8000 B2 with Nanofillers In order to shift the viscosity into the printable range, NPs were incorporated in differ- ent amounts. Therefore PEG 8000 B2 was investigated with varying amounts of nanofillers: In order to shift the viscosity into the printable range, NPs were incorporated in dif- NP–alk (15–17) and NP–OH (nanopowder, 18–20). The rheology results are given in Fig- ferent amounts. Therefore PEG 8000 B2 was investigated with varying amounts of nano- ure 7. Samples 15–20 exhibit a drastic increase in viscosity even at higher temperatures. Sample 17 can be applicable for 3D printing as the viscosity vs shear rate is fitting into fillers: NP-alk (15–17) and NP-OH (nanopowder, 18–20). The rheology results are given the desired range. Polymer composites in Figure 7b show shear thinning behavior which in Figure 7. Samples 15-20 exhibit a drastic increase in viscosity even at higher tempera- reduces viscosity below required values. Samples 11–13 (Figure 7c) containing LiTFSI with tures. Sample 17 can be applicable for 3D printing as the viscosity vs shear rate is fitting different ratios of the NP–alk also display a shear depended viscosity and will require more adjustment for performing 3D printing on our setup, as the viscosity values at low into the desired range. Polymer composites in Figure 7b show shear thinning behavior shear rate are too high to have the polymer melt flow from storage tank to printing head. which reduces viscosity below required values. Samples 11–13 (Figure 7c) containing Sample 14 (Figure 7d) containing additional NP–IL shows printability but at relatively low LiTFSI with different ratios of the NP-a◦lk also display a shear depended viscosity and will temperatures (20–40 C) which can be problematic as printing carried out close to room require more adjustment for performing 3D printing on our setup, as the viscosity values temperature is less likely to keep the desired shape. PreviouslywereportedthatPEG1500UPy assuchisnotsuitablefor3Dprintingin 2 at low shear rate are too high to have the polymer melt flow from storage tank to printing our 3D-printer without the use of additional fillers [28]. Figure 8 represents the rheological head. Sample 14 (Figure 7d) containing additional NP-IL shows printability but at rela- measurements of PEG 1500 UPy2/LiTFSI now containing either NP–IL (2,3,4) or the NP– tively low temperaturOesH(2(50,6–,74)0, s°hCow) winghincchrecaasendbveispcorsoitbylaenmd atlsico alinseparibnethianvgiocr aorfrsiheedarovustvcislcooseity. Thus, the presented composite materials exhibit promising properties suitable for 3D to room temperature is less likely to keep the desired shape. printing process. Previously we reported that PEG 1500 UPy2 as such is not suitable for 3D printing in our 3D-printer without the use of additional fillers [28]. Figure 8 represents the rheological measurements of PEG 1500 UPy2/LiTFSI now containing either NP-IL (2,3,4) or the NP- OH (5,6,7), showing increased viscosity and also linear behavior of shear vs viscosity. Thus, the presented composite materials exhibit promising properties suitable for 3D printing process. 2 ◦◦PDF Image | 3D Printable Composite Polymer Electrolytes
PDF Search Title:
3D Printable Composite Polymer ElectrolytesOriginal File Name Searched:
nanomaterials-12-01859-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |