
PDF Publication Title:
Text from PDF Page: 004
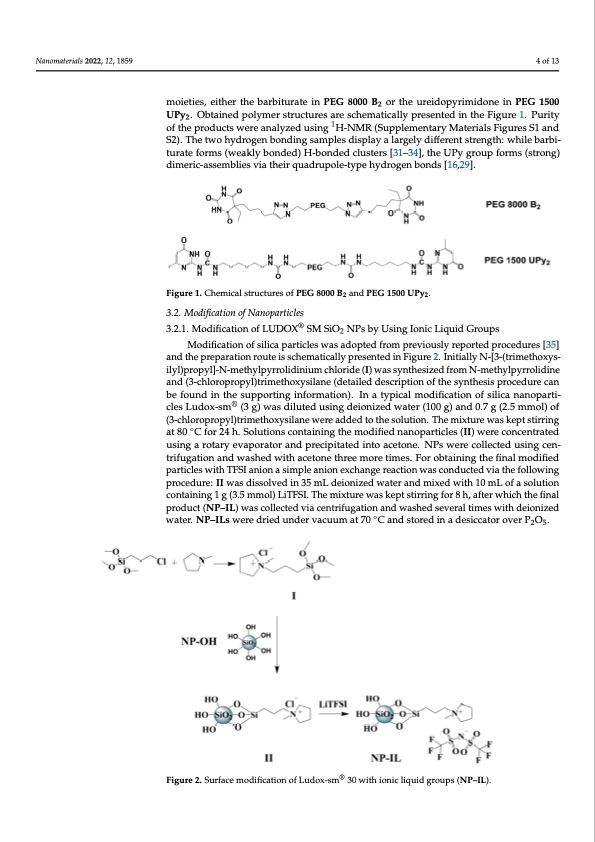
Nanomaterials 2022, 12, x FOR PEER REVIEW 4 of 14 Nanomaterials 2022, 12, 1859 3. Results and Discussions 3.1. Modification of PEG Polymers 4 of 13 PEG 1500 and PEG 8000 were end group modified according to already known pro- cedures [20,29,30], in order to obtain telechelic polymers containing hydrogen bonding moieties, eiittherrththeebabrabribtiuturartaeteiniPnEPGEG800800B02 oBr thoer uthreiduorpeiydroimpyidriomneidinonPeEGin 1P5E0G0 U1P5y002. 2 UOPbytai.nOedbtpaoinlyemdeproslytrmucetrusrtersucatruersecshaermeastcihcaelmlyatpicraeslleynpterdesienntehdeiFnigtuhreeF1ig.uPrueri1t.yPoufrtihtye 2 als 2022, 12, x FOR PEER REVIEW via the following procedure: II was dissolved in 35 mL deionized water and mixed with 5 of 14 of the products were analyzed using 1H-NMR (Supplementary Materials Figures S1 and products were analyzed using 1H-NMR (Supplementary Materials Figures S1 and S2). The S2). The two hydrogen bonding samples display a largely different strength: while barbi- two hydrogen bonding samples display a largely different strength: while barbiturate turate forms (weakly bonded) H-bonded clusters [31–34], the UPy group forms (strong) forms (weakly bonded) H-bonded clusters [31–34], the UPy group forms (strong) dimeric- dimeric-assemblies via their quadrupole-type hydrogen bonds [16,29]. assemblies via their quadrupole-type hydrogen bonds [16,29]. Figure 1. Chemical structures of PEG 8000 B2 and PEG 1500 UPy2. Figure 1. Chemical structures of PEG 8000 B2 and PEG 1500 UPy2. 3.2. Modification of Nanoparticles 3.2. Modification of Nanoparticle®s 3.2.1. Modification of LUDOX SM SiO2 NPs by Using Ionic Liquid Groups 3.2.1. Modification of LUDOX® SM SiO2 NPs by Using Ionic Liquid Groups . Modification of silica particles was adopted from previously reported procedures [35] and thMeopdriefpicartaiotinonorfosuitleicias spcahretmiclaetsicwalalyspardeosepnteteddfirnoFmigpurev2i.oIunsitliyalrleypNo-r[t3e-d(trpimroectehdouxryess- i[l3y5l)]parnodpytlh]-eNp-rmepetahryaltpioynrroroliudtieniusmscchhelmoraidtieca(Ill)ywparsesyentheedsiznedFifgruomre N2.-mIneithiayllpyyNrr-o[3li-d(tinrie- amnedth(3o-xcyhsloilryol)pprrooppyyl)lt]r-iNm-emthetohxylspilyarnreol(ideintaiuilemddcehslcoriipdteion(Io)fwthaessysnytnhtehseisipzerodcefdruomrecNan- bmeeftohuynlpdyrinrotlihdeinseuapnpdor(t3i-ncghlionrfoprmroaptyiol)ntr)i.mIenthaotxyypsiclanl em(oddetiafiicleadtiodnesocfrispitliocan nofanthoepsayrnti- cles Ludox-sm® (3 g) was diluted using deionized water (100 g) and 0.7 g (2.5 mmol) of thesis procedure can be found in the supporting information). In a typical modification of (3-chloropropyl)trimethoxysilan®ewereaddedtothesolution.Themixturewaskeptstirring silica nanoparticles Ludox-sm (3 g) was diluted using deionized water (100 g) and 0.7 g at 80 ◦C for 24 h. Solutions containing the modified nanoparticles (II) were concentrated (2.5 mmol) of (3-chloropropyl)trimethoxysilane were added to the solution. The mixture using a rotary evaporator and precipitated into acetone. NPs were collected using cen- was kept stirring at 80 °C for 24 h. Solutions containing the modified nanoparticles (II) trifugation and washed with acetone three more times. For obtaining the final modified were concentrated using a rotary evaporator and precipitated into acetone. NPs were col- particles with TFSI anion a simple anion exchange reaction was conducted via the following lected using centrifugation and washed with acetone three more times. For obtaining the procedure: II was dissolved in 35 mL deionized water and mixed with 10 mL of a solution final modified particles with TFSI anion a simple anion exchange reaction was conducted containing 1 g (3.5 mmol) LiTFSI. The mixture was kept stirring for 8 h, after which the final product (NP–IL) was collected via centrifugation and washed several times with deionized 10 mL of a solution containing 1 g (3.5 mmol) LiTFSI. The mixture was kept stirring for 8 water. NP–ILs were dried under vacuum at 70 ◦C and stored in a desiccator over P2O5. h, after which the final product (NP-IL) was collected via centrifugation and washed sev- eral times with deionized water. NP-ILs were dried under vacuum at 70 °C and stored in a desiccator over P2O5. ®® Figure 2. SurfacFeigmuroed2i.fiScuartfiaocne omfoLduifidcoaxti-osnmof 3L0udwoixt-hsmioni3c0 lwiqiuthidiognricouliqpusid(NgPro-uILp)s. (NP–IL). 3.2.2. Modification of Silica Nanopowder Nanopowder was modified with (dodecyl)dimethylsilane groups which is schemat- ically presented in Figure 3. 1 g of previously dried (170 °C for 72 h under high vacuum)PDF Image | 3D Printable Composite Polymer Electrolytes
PDF Search Title:
3D Printable Composite Polymer ElectrolytesOriginal File Name Searched:
nanomaterials-12-01859-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |