
PDF Publication Title:
Text from PDF Page: 004
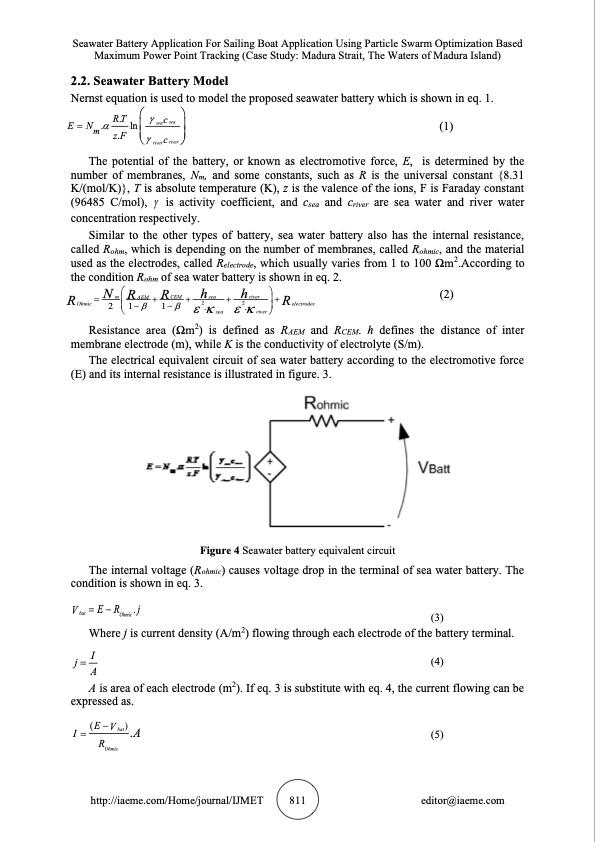
Seawater Battery Application For Sailing Boat Application Using Particle Swarm Optimization Based Maximum Power Point Tracking (Case Study: Madura Strait, The Waters of Madura Island) 2.2. Seawater Battery Model Nernst equation is used to model the proposed seawater battery which is shown in eq. 1. R.T c E=N. lnseasea (1) m z.F c river river The potential of the battery, or known as electromotive force, E, is determined by the number of membranes, Nm, and some constants, such as R is the universal constant {8.31 K/(mol/K)}, T is absolute temperature (K), z is the valence of the ions, F is Faraday constant (96485 C/mol), is activity coefficient, and csea and criver are sea water and river water concentration respectively. Similar to the other types of battery, sea water battery also has the internal resistance, called Rohm, which is depending on the number of membranes, called Rohmic, and the material used as the electrodes, called Relectrode, which usually varies from 1 to 100 Ωm2.According to the condition Rohm of sea water battery is shown in eq. 2. NRRhhR m AEM + CEM + sea + river + (2) Resistance area (Ωm2) is defined as RAEM and RCEM. h defines the distance of inter R The electrical equivalent circuit of sea water battery according to the electromotive force (E) and its internal resistance is illustrated in figure. 3. Figure 4 Seawater battery equivalent circuit The internal voltage (Rohmic) causes voltage drop in the terminal of sea water battery. The = Ohmic 2 2 electrodes 21−1−. . river membrane electrode (m), while K is the conductivity of electrolyte (S/m). condition is shown in eq. 3. Vbat =E−ROhmic.j sea (3) Where j is current density (A/m2) flowing through each electrode of the battery terminal. j=I (4) A A is area of each electrode (m2). If eq. 3 is substitute with eq. 4, the current flowing can be expressed as. I = (E−Vbat).A (5) ROhmic http://iaeme.com/Home/journal/IJMET 811 editor@iaeme.comPDF Image | SEAWATER BATTERY APPLICATION FOR SAILING BOAT
PDF Search Title:
SEAWATER BATTERY APPLICATION FOR SAILING BOATOriginal File Name Searched:
IJMET_10_01_083.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |