
PDF Publication Title:
Text from PDF Page: 048
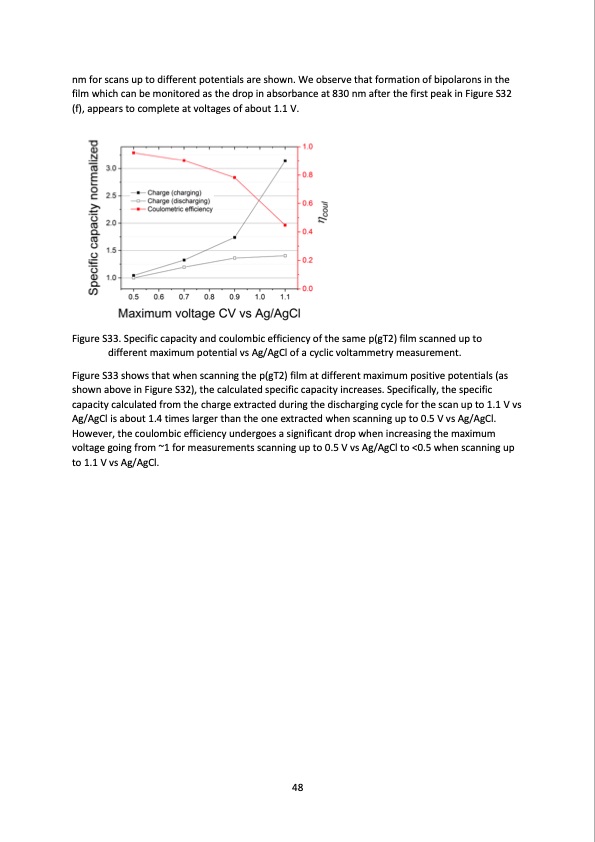
nm for scans up to different potentials are shown. We observe that formation of bipolarons in the film which can be monitored as the drop in absorbance at 830 nm after the first peak in Figure S32 (f), appears to complete at voltages of about 1.1 V. Figure S33. Specific capacity and coulombic efficiency of the same p(gT2) film scanned up to different maximum potential vs Ag/AgCl of a cyclic voltammetry measurement. Figure S33 shows that when scanning the p(gT2) film at different maximum positive potentials (as shown above in Figure S32), the calculated specific capacity increases. Specifically, the specific capacity calculated from the charge extracted during the discharging cycle for the scan up to 1.1 V vs Ag/AgCl is about 1.4 times larger than the one extracted when scanning up to 0.5 V vs Ag/AgCl. However, the coulombic efficiency undergoes a significant drop when increasing the maximum voltage going from ~1 for measurements scanning up to 0.5 V vs Ag/AgCl to <0.5 when scanning up to 1.1 V vs Ag/AgCl. 48PDF Image | salt water battery with high stability
PDF Search Title:
salt water battery with high stabilityOriginal File Name Searched:
salt-water-battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |