
PDF Publication Title:
Text from PDF Page: 004
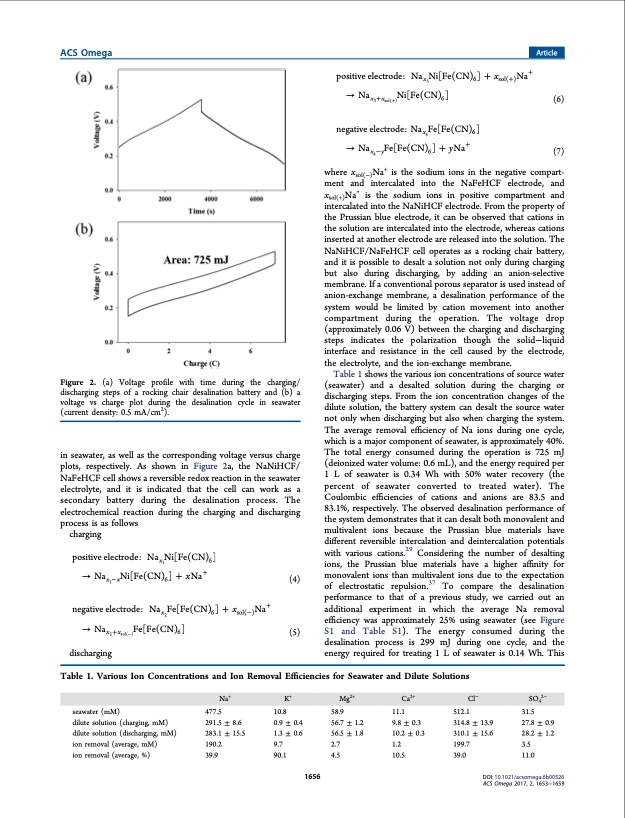
ACS Omega Article Figure 2. (a) Voltage profile with time during the charging/ discharging steps of a rocking chair desalination battery and (b) a voltage vs charge plot during the desalination cycle in seawater (current density: 0.5 mA/cm2). in seawater, as well as the corresponding voltage versus charge plots, respectively. As shown in Figure 2a, the NaNiHCF/ NaFeHCF cell shows a reversible redox reaction in the seawater electrolyte, and it is indicated that the cell can work as a secondary battery during the desalination process. The electrochemical reaction during the charging and discharging process is as follows charging positiveelectrode: Nax1Ni[Fe(CN)6] positive electrode: Nax3Ni[Fe(CN)6] + xsol(+)Na+ → Nax3+xsol(+)Ni[Fe(CN)6] (6) negative electrode: Nax4Fe[Fe(CN)6] → Nax4−yFe[Fe(CN)6] + yNa+ (7) where xsol(−)Na+ is the sodium ions in the negative compart- ment and intercalated into the NaFeHCF electrode, and xsol(+)Na+ is the sodium ions in positive compartment and intercalated into the NaNiHCF electrode. From the property of the Prussian blue electrode, it can be observed that cations in the solution are intercalated into the electrode, whereas cations inserted at another electrode are released into the solution. The NaNiHCF/NaFeHCF cell operates as a rocking chair battery, and it is possible to desalt a solution not only during charging but also during discharging, by adding an anion-selective membrane. If a conventional porous separator is used instead of anion-exchange membrane, a desalination performance of the system would be limited by cation movement into another compartment during the operation. The voltage drop (approximately 0.06 V) between the charging and discharging steps indicates the polarization though the solid−liquid interface and resistance in the cell caused by the electrode, the electrolyte, and the ion-exchange membrane. Table 1 shows the various ion concentrations of source water (seawater) and a desalted solution during the charging or discharging steps. From the ion concentration changes of the dilute solution, the battery system can desalt the source water not only when discharging but also when charging the system. The average removal efficiency of Na ions during one cycle, which is a major component of seawater, is approximately 40%. The total energy consumed during the operation is 725 mJ (deionized water volume: 0.6 mL), and the energy required per 1 L of seawater is 0.34 Wh with 50% water recovery (the percent of seawater converted to treated water). The Coulombic efficiencies of cations and anions are 83.5 and 83.1%, respectively. The observed desalination performance of the system demonstrates that it can desalt both monovalent and multivalent ions because the Prussian blue materials have different reversible intercalation and deintercalation potentials with various cations.29 Considering the number of desalting ions, the Prussian blue materials have a higher affinity for monovalent ions than multivalent ions due to the expectation → Nax −xNi[Fe(CN)6] + xNa+ (4) 1 of electrostatic repulsion.37 To compare the desalination negative electrode: Nax2Fe[Fe(CN)6] + xsol(−)Na+ → Nax2+xsol(−)Fe[Fe(CN)6] (5) discharging performance to that of a previous study, we carried out an additional experiment in which the average Na removal efficiency was approximately 25% using seawater (see Figure S1 and Table S1). The energy consumed during the desalination process is 299 mJ during one cycle, and the energy required for treating 1 L of seawater is 0.14 Wh. This Table 1. Various Ion Concentrations and Ion Removal Efficiencies for Seawater and Dilute Solutions Na+ K+ Mg2+ Ca2+ Cl− SO42− seawater (mM) dilute solution (charging, mM) dilute solution (discharging, mM) ion removal (average, mM) ion removal (average, %) 477.5 291.5 ± 8.6 283.1 ± 15.5 190.2 39.9 10.8 0.9 ± 0.4 1.3 ± 0.6 9.7 90.1 58.9 56.7 ± 1.2 56.5 ± 1.8 2.7 4.5 11.1 9.8 ± 0.3 10.2 ± 0.3 1.2 10.5 512.1 314.8 310.1 199.7 39.0 ± 13.9 ± 15.6 31.5 27.8 ± 0.9 28.2 ± 1.2 3.5 11.0 1656 DOI: 10.1021/acsomega.6b00526 ACS Omega 2017, 2, 1653−1659PDF Image | Rocking Chair Desalination Battery Prussian Blue Electrodes
PDF Search Title:
Rocking Chair Desalination Battery Prussian Blue ElectrodesOriginal File Name Searched:
rocking-chair-desalination-battery-prussian-blue.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |