
PDF Publication Title:
Text from PDF Page: 003
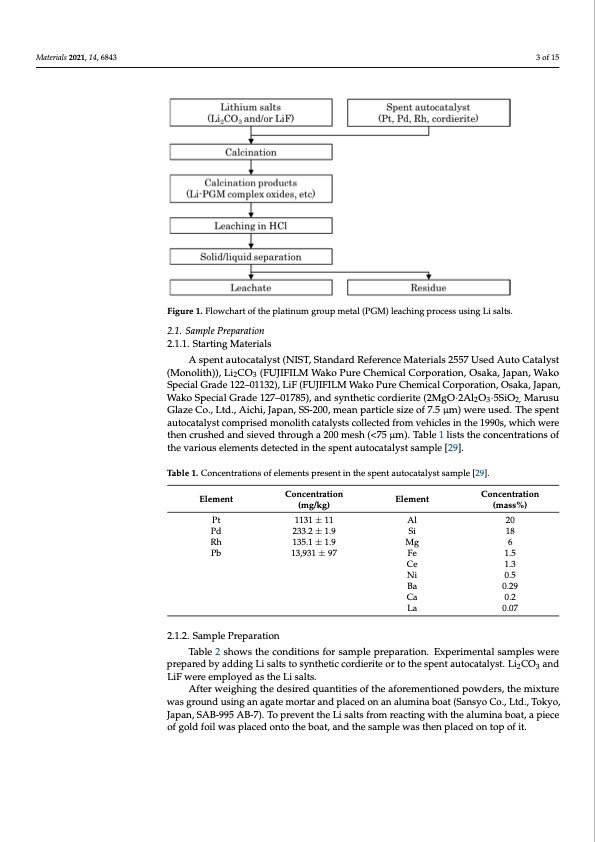
Materials 2021, 14, x FOR PEER REVIEW 3 of 16 Materials 2021, 14, 6843 2. Materials and Methods Figure 1 shows the flowchart of the leaching process. Herein, PGM recovery experi- ments from spent autocatalysts were conducted using lithium carbonate (Li2CO3) and lith- ium fluoride (LiF) as Li sources. Figure 1. Flowchart of the platinum group metal (PGM) leaching process using Li salts. Figure 1. Flowchart of the platinum group metal (PGM) leaching process using Li salts. 2.1. Sample Preparation 2.1. Sample Preparation 2.1.1. Starting Materials 2.1.1. Starting Materials A spent autocatalyst (NIST, Standard Reference Materials 2557 Used Auto Catalyst A spent autocatalyst (NIST, Standard Reference Materials 2557 Used Auto Catalyst (Monolith)), Li2CO3 (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan, Wako (Monolith)), Li2CO3 (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan, Wako Pt 1131 ± 11 Al 20 Pd 233.2 ± 1.9 Si 18 SiMg 186 PdRh 23133.25.1± ±1.91.9 13,931 ± 97 135.1 ± 1.9 13,931 ± 97 Rh Pb Mg 6 Pb Fe 1.5 LiF were employed as the Li salts. (mg/kg) (mg/kg) (mass%) 3 of 15 Special Grade 122–01132), LiF (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan, Special Grade 122–01132), LiF (FUJIFILM Wako Pure Chemical Corporation, Osaka, Ja- Wako Special Grade 127–01785), and synthetic cordierite (2MgO·2Al2O3·5SiO2, Marusu pan,Wako Special Grade 127–01785), and synthetic cordierite (2MgO·2Al2O3·5SiO2, Glaze Co., Ltd., Aichi, Japan, SS-200, mean particle size of 7.5 μm) were used. The spent Marusu Glaze Co., Ltd., Aichi, Japan, SS-200, mean particle size of 7.5 μm) were used. The autocatalyst comprised monolith catalysts collected from vehicles in the 1990s, which were spent autocatalyst comprised monolith catalysts collected from vehicles in the 1990s, then crushed and sieved through a 200 mesh (<75 μm). Table 1 lists the concentrations of which were then crushed and sieved through a 200 mesh (<75 μm). Table 1 lists the con- the various elements detected in the spent autocatalyst sample [29]. centrations of the various elements detected in the spent autocatalyst sample [29]. Table 1. Concentrations of elements present in the spent autocatalyst sample [29]. Table 1. Concentrations of elements present in the spent autocatalyst sample [29]. Element Element Pt Concentration Element Concentration Concentration Element (mass%) Concentration 1131 ± 11 Al 20 Table 2 shows the conditions for sample preparation. Experimental samples were After weighing the desired quantities of the aforementioned powders, the mixture prepared by adding Li salts to synthetic cordierite or to the spent autocatalyst. Li2CO3 and was ground using an agate mortar and placed on an alumina boat (Sansyo Co., Ltd., Tokyo, LiF were employed as the Li salts. Japan, SAB-995 AB-7). To prevent the Li salts from reacting with the alumina boat, a piece of gold foil was placed onto the boat, and the sample was then placed on top of it. Ce 1.3 Fe 1.5 Ni 0.5 Ce 1.3 Ba 0.29 NCia 0.50.2 La 0.07 2.1.2. Sample Preparation Ba 0.29 Ca 0.2 La 0.07 Table 2 shows the conditions for sample preparation. Experimental samples were p2r.e1p.2a.rSeadmbpylaedPdrienpgaLraitsioalnts to synthetic cordierite or to the spent autocatalyst. Li2CO3 andPDF Image | Recovery of Platinum Group Metals from Auto Catalysts
PDF Search Title:
Recovery of Platinum Group Metals from Auto CatalystsOriginal File Name Searched:
materials-14-06843-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |