
PDF Publication Title:
Text from PDF Page: 007
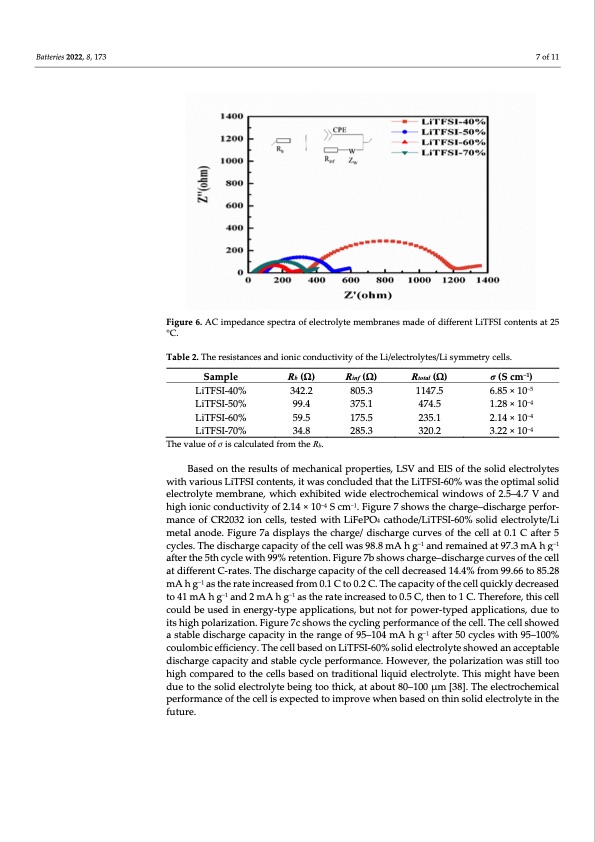
Batteries 2022, 8, 173 7 of 11 Figure 6. AC impedance spectra of electrolyte membranes made of different LiTFSI contents at 25 °C. Table 2. The resistances and ionic conductivity of the Li/electrolytes/Li symmetry cells. Sample LiTFSI-40% LiTFSI-50% LiTFSI-60% LiTFSI-70% Rb (Ω) 342.2 99.4 59.5 34.8 Rinf (Ω) 805.3 375.1 175.5 285.3 Rtotal (Ω) 1147.5 474.5 235.1 320.2 σ (S cm−1) 6.85 × 10−5 1.28 × 10−4 2.14 × 10−4 3.22 × 10−4 The value of σ is calculated from the Rb. Based on the results of mechanical properties, LSV and EIS of the solid electrolytes with various LiTFSI contents, it was concluded that the LiTFSI-60% was the optimal solid electrolyte membrane, which exhibited wide electrochemical windows of 2.5–4.7 V and high ionic conductivity of 2.14 × 10−4 S cm−1. Figure 7 shows the charge–discharge perfor- mance of CR2032 ion cells, tested with LiFePO4 cathode/LiTFSI-60% solid electrolyte/Li metal anode. Figure 7a displays the charge/ discharge curves of the cell at 0.1 C after 5 cycles. The discharge capacity of the cell was 98.8 mA h g−1 and remained at 97.3 mA h g−1 after the 5th cycle with 99% retention. Figure 7b shows charge–discharge . The discharge capacity of the cell 99.66 to 85.28 mA h g−1 a he capacity of the cell quickly decreased to41mAhg−1 and2mAhg−1 a .Therefore,thiscell could be used in energy-type applications, but not for power-typed applications, due to its high polarization. Figure 7c shows the cycling performance of the cell. The cell showed a stable discharge capacity in the range of 95–104 mA h g−1 after 50 cycles with 95–100% coulombic efficiency. The cell based on LiTFSI-60% solid electrolyte showed an acceptable discharge capacity and stable cycle performance. However, the polarization was still too high compared to the cells based on traditional liquid electrolyte. This might have been due to the solid electrolyte being too thick, at about 80–100 μm [38]. The electrochemical performance of the cell is expected to improve when based on thin solid electrolyte in the future. at different C-rates curves of the cell decreased 14.4% from s the rate increased from 0.1 C to 0.2 C. T s the rate increased to 0.5 C, then to 1 CPDF Image | Lithium Salt Concentration on Materials
PDF Search Title:
Lithium Salt Concentration on MaterialsOriginal File Name Searched:
batteries-08-00173.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |