
PDF Publication Title:
Text from PDF Page: 003
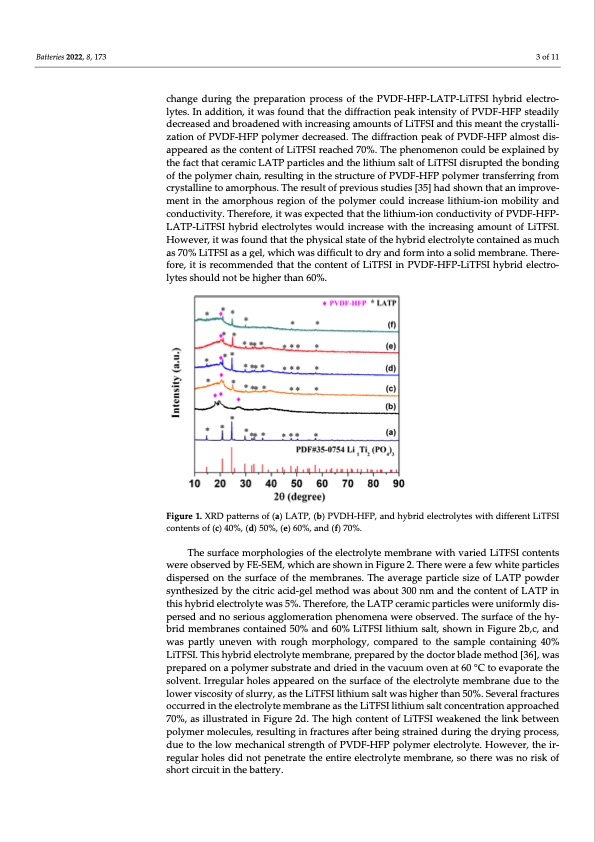
Batteries 2022, 8, 173 3 of 11 change during the preparation process of the PVDF-HFP-LATP-LiTFSI hybrid electro- lytes. In addition, it was found that the diffraction peak intensity of PVDF-HFP steadily decreased and broadened with increasing amounts of LiTFSI and this meant the crystalli- zation of PVDF-HFP polymer decreased. The diffraction peak of PVDF-HFP almost dis- appeared as the content of LiTFSI reached 70%. The phenomenon could be explained by the fact that ceramic LATP particles and the lithium salt of LiTFSI disrupted the bonding of the polymer chain, resulting in the structure of PVDF-HFP polymer transferring from crystalline to amorphous. PVDF-HFP- LATP-LiTFSI hybrid electrolytes would increase with the increasing amount of LiTFSI. hybrid electrolyte contained as much conductivity. Therefore, it was expected that the lithium-ion conductivity of However, it was found that the physical state of the as 70% LiTFSI as a gel lytes PVDF-HFP-LiTFSI hybrid electro- The result of previous studies [35] had shown that an improve- ment in the amorphous region of the polymer could increase lithium-ion mobility and , which was difficult to dry and form into a solid membrane. There- fore, it is recommended that the content of LiTFSI in should not be higher than 60%. Figure 1. XRD patterns of (a) LATP, (b) PVDH-HFP, and hybrid electrolytes with different LiTFSI contents of (c) 40%, (d) 50%, (e) 60%, and (f) 70%. The surface morphologies of the electrolyte membrane with varied LiTFSI contents were observed by FE-SEM, which are shown in Figure 2. There were a few white particles dispersed on the surface of the membranes. The average particle size of LATP powder synthesized by the citric acid-gel method was about 300 nm and the content of LATP in this hybrid electrolyte was 5%. Therefore, the LATP ceramic particles were uniformly dis- persed and no serious agglomeration phenomena were observed. The surface of the hy- brid membranes contained 50% and 60% LiTFSI lithium salt, shown in Figure 2b,c, and was partly uneven with rough morphology, compared to the sample containing 40% LiTFSI. This hybrid electrolyte membrane, prepared by the doctor blade method [36], was prepared on a polymer substrate and dried in the vacuum oven at 60 °C to evaporate the solvent. Irregular holes appeared on the surface of the electrolyte membrane due to the lower viscosity of slurry, as the LiTFSI lithium salt was higher than 50%. Several fractures occurred in the electrolyte membrane as the LiTFSI lithium salt concentration approached 70%, as illustrated in Figure 2d. The high content of LiTFSI weakened the link between polymer molecules, resulting in fractures after being strained during the drying process, due to the low mechanical strength of PVDF-HFP polymer electrolyte. However, the ir- regular holes did not penetrate the entire electrolyte membrane, so there was no risk of short circuit in the battery.PDF Image | Lithium Salt Concentration on Materials
PDF Search Title:
Lithium Salt Concentration on MaterialsOriginal File Name Searched:
batteries-08-00173.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |