
PDF Publication Title:
Text from PDF Page: 008
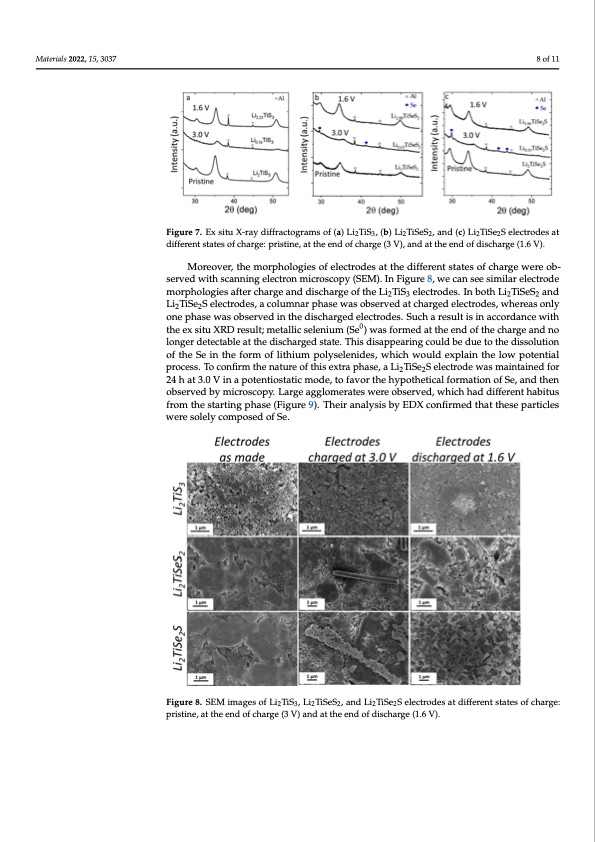
Materials 2022, 15, x FOR PEER REVIEW Materials 2022, 15, x FOR PEER REVIEW Materials 2022, 15, 3037 9 of 12 Figure 7. Ex situ X-ray diffractograms of (a) Li TiS , (b) Li TiSeS , and (c) Li TiSe S electrodes at different states of charge: pristine, at the end of c2har3ge (3 V),2 and a2t the end of 2disch2arge (1.6 V). different states of charge: pristine, at the end of charge (3 V), and at the end of discharge (1.6 V different states of charge: pristine, at the end of charge (3 V), and at the end of discharge (1.6 V). Moreover, the morphologies of electrodes at the different states of charge were ob- Moreover, the morphologies of electrodes at the different states of charge were 8 of 11 9 Figure 7. Ex situ X-ray diffractograms of (a) Li2TiS3, (b) Li2TiSeS2, and (c) Li2TiSe2S electrodes at Figure 7. Ex situ X-ray diffractograms of (a) Li2TiS3, (b) Li2TiSeS2, and (c) Li2TiSe2S electrod Moreover, the morphologies of electrodes at the different states of charge were ob- served with scanning electron microscopy (SEM). In Figure 8, we can see similar electrode served with scanning electron microscopy (SEM). In Figure 8, we can see similar electr served with scanning electron microscopy (SEM). In Figure 8, we can see similar electrode morphologies after charge and discharge of the Li2TiS3 electrodes. In both Li2TiSeS2 and morphologies after charge and discharge of the Li2TiS3 electrodes. In both Li2TiSeS2 morphologies after charge and discharge of the Li2TiS3 electrodes. In both Li2TiSeS2 and Li2TiSe2S electrodes, a columnar phase was observed at charged electrodes, whereas only Li2TiSe2S electrodes, a columnar phase was observed at charged electrodes, whereas Li2TiSe2S electrodes, a columnar phase was observed at charged electrodes, whereas only one phase was observed in the discharged electrodes. Such a result is in accordance with one phase was observed in the discharged electrodes. Such a result is in accordance one phase was observed in the discharged electrodes. Such a result is in accordance with the ex situ XRD result; metallic selenium (Se0) was formed0at the end of the charge and no the ex situ XRD result; metallic selenium (Se ) was formed at the end of the charge an the ex situ XRD result; metallic selenium (Se0) was formed at the end of the charge and no longer detectableonagtetrhededtiesccthabalregeadt tshteatdei.sTchaisrgdeidsasptapte.arTihnigs cdoisualpdpbeeardinugectoutlhdebdeisdsuoeluto- the diss longer detectable at the discharged state. This disappearing could be due to the dissolution tion of the Se in tihoenfofrtmheoSfeliitnhituhme fopromlyosef leitnhiiduems,pwohlyicsehlewnoiduelsd, wexhpilcahinwtohueldloewxploaitnenthtiealow pote of the Se in the form of lithium polyselenides, which would explain the low potential process. To confpiromcetshse. Tnoatcuornefiorfmththiseenxattruarpehoafsteh,isaeLxit2rTaiSpeh2aSse,leactLrio2TdieSew2Saselmecatrinotdaeinweads maintai process. To confirm the nature of this extra phase, a Li2TiSe2S electrode was maintained for for 24 h at 3.0 Vfoinr 2a4phoatetn3t.i0osVtaitnicampotdeen,titostfatvicormtohdeeh, ytopofathvoetricthale fhoyrpmoatthieotnicaolf fSoer,maantdion of Se, 24 h at 3.0 V in a potentiostatic mode, to favor the hypothetical formation of Se, and then then observed bthyemn iocbrosesrcvoepdy.bLyamrgiceroasgcgolpoym. eLrartgees wagegrleomobesraetrevsedw,ewrehoicbhsehrvaeddd, iwffheircehnthad diffe observed by microscopy. Large agglomerates were observed, which had different habitus habitus from the starting phase (Figure 9). Their analysis by EDX confirmed that t habitus from the starting phase (Figure 9). Their analysis by EDX confirmed that these from the starting phase (Figure 9). Their analysis by EDX confirmed that these particles particles were solely composed of Se. particles were solely composed of Se. were solely composed of Se. Figure 8. SEM images of Li2TiS3, Li2TiSeS2, and Li2TiSe2S electrodes at different states of ch pristine, at the end of charge (3 V) and at the end of discharge (1.6 V). Figure 8. SEM images of Li2TiS3, Li2TiSeS2, and Li2TiSe2S electrodes at different states of charge: Figure 8. SEM images of Li2TiS3, Li2TiSeS2, and Li2TiSe2S electrodes at different states of charge: pristine, at the end of charge (3 V) and at the end of discharge (1.6 V). pristine, at the end of charge (3 V) and at the end of discharge (1.6 V). o e ) o w d o n r h aPDF Image | Lithium-Rich Rock Salt Type Sulfides-Selenides
PDF Search Title:
Lithium-Rich Rock Salt Type Sulfides-SelenidesOriginal File Name Searched:
materials-15-03037-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |