
PDF Publication Title:
Text from PDF Page: 005
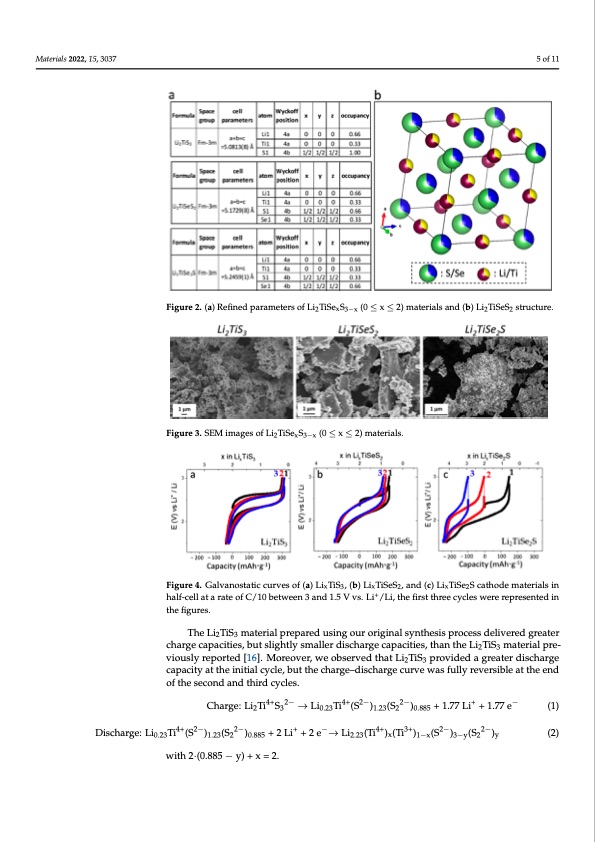
® conitfyiromf itnhge mthaeteSreiasluabnsdtituhteiopnre.sFeonrctehoefRaibertvoaedldsrigefnianlecmomenintsg, ofrwomingthteoKthaeptpoonorpcrroytsetcatlilvien- Materials 2022, 15, 3037 pattern was already quite good and confirmed that Se substitution had occurred in these samples. 5 of 11 Figure 3. SEM images of Li2TiSexS3−x (0 ≤ x ≤ 2) materials. ously, the Li2TiSeS2 agglomerates tended to have a plate-like morphology. ® ityfiolfmt,hiet mwastedrieaclidaendttohenoptreresfeinceethoef atbormoaicdoscicgunpaalnccoimesinangdfrkoemepththeeKmapotdoenl stprurocteucrteisv.e However, without further refining, the agreement between the observed and calculated film, it was decided to not refine the atomic occupancies and keep the model structures. pattern was already quite good and confirmed that Se substitution had occurred in these However, without further refining, the agreement between the observed and calculated samples. Figure 2. (a) Refined parameters of Li2TiSexS3−x (0 ≤ x ≤ 2) materials and (b) Li2TiSeS2 structure. Figure 2T. h(ae) FRiegfuinred3psahroamwestSerEsMofiLmi2aTgiSesexoSf3−Lx (i02T≤iSxe≤xS23)−xmpaotwerdiaelsrsa.nd (b) Li2TiSeS2 structure. Figure 2. (a) Refined parameters of Li2TiSexS3−x (0 ≤ x ≤ 2) materials and (b) Li2TiSeS2 structure. The Figure 3 shows SEM images of Li2TiSexS3−x powders. Materials 2022, 15, x FOR PEER REVIEW 6 of 12 Figure 3. SEM images of Li2TiSexS3−x (0 ≤ x ≤ 2) materials. Figure 3. SEM images of Li2TiSexS3−x (0 ≤ x ≤ 2) materials. All powders were composed of agglomerates of primary nanoparticles, and, curi- The electrochemical performances of Li2TiS3 and selenium-substituted samples All powders were composed of agglomerates of primary nanoparticles, and, curi- (Li2TiSeS2 and Li2TiSe2S) were investigated and compared in half-cells (Figure 4). ously, the Li2TiSeS2 agglomerates tended to have a plate-like morphology. The electrochemical performances of Li2TiS3 and selenium-substituted samples (Li2TiSeS2 and Li2TiSe2S) were investigated and compared in half-cells (Figure 4). Figure 4. Galvanostatic curves of (a) LixTiS3, (b) LixTiSeS2, and (c) LixTiSe2S cathode materials in Figure 4. Galvanostatic curves of (a) LixTiS3, (b) LixTiSeS2, and (c) LixTiSe2S cathode materials in half- half-cell at a rate of C/10 between 3 and 1.5 V vs.+Li+/Li, the first three cycles were represented in cell at a rate of C/10 between 3 and 1.5 V vs. Li /Li, the first three cycles were represented in the the figures. figures. TheLiTiS materialpreparedusingouroriginalsynthesisprocessdeliveredgreater 23 The open circuit potential of Li2TiS3 was measured as 2.31 V at the beginning of the chargecapacities,butslightlysmallerdischargecapacities,thantheLiTiS materialpre- 23+ cycling. When the Li2TiS3 was charged with a C-rate of C/10 until 3 V, the 1.77 Li ions viously reported [16]. Moreover, we observed that Li TiS provided a greater discharge 2 3−1 were extracted from Li2TiS3 and a capacity of 300 mAh·g was delivered. During dis- capacity at the initial cycle, but the charge–discharge curve was fully reversible at the end charge, 2.0 Li+ ions were inserted into the structure, the composition changed into of the second and third cycles. Li2.23TiS3, and a capacity of 339 mAh·g−1 was delivered. The average charge and discharge potentials were measured at 2.46 V and 2.23 V, respectively, and a summary of the results Charge: Li2Ti4+S32− → Li0.23Ti4+(S2−)1.23(S22−)0.885 + 1.77 Li+ + 1.77 e− (1) is shown in Table 1. Discharge: Li0.23Ti4+(S2−)1.23(S22−)0.885 + 2 Li+ + 2 e−→ Li2.23(Ti4+)x(Ti3+)1−x(S2−)3−y(S22−)y (2) Table 1. Summary of electrochemical performances of Li2TiSexS3−x (0 ≤ x ≤ 2) materials. with 2·(0.885 − y) + x = 2. 1st Charge Capacity (mAh·g−1) 300 179 149 1st Dis- Average charge Ca- Charge Poten- Average Dis- charge Potential (V vs. Li+/Li) 2.23 2.06 1.98 Composition Li2TiS3 Li2TiSeS2 Li2TiSeS2 Lattice Pa- rameter a (Å) 5.0831 5.1729 5.2459 pacity (mAh·g−1) 339 310 379 tial (V vs. Li+/Li) 2.46 2.34 2.24PDF Image | Lithium-Rich Rock Salt Type Sulfides-Selenides
PDF Search Title:
Lithium-Rich Rock Salt Type Sulfides-SelenidesOriginal File Name Searched:
materials-15-03037-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |