
PDF Publication Title:
Text from PDF Page: 012
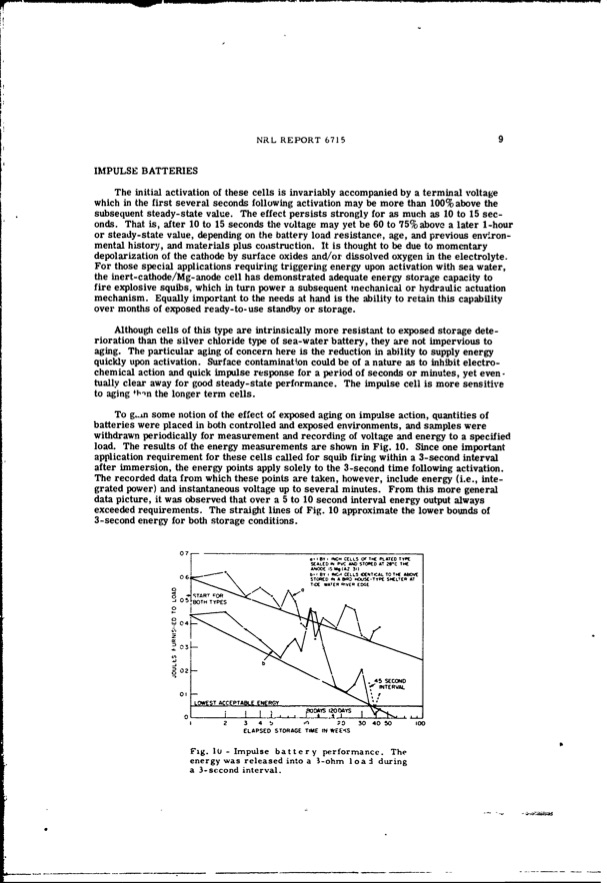
IMPULSE BATTERIES oo o 03 07 06 S:TA.RT T=IOYRPETR aI I% INCHCELLS Of lt~ PLATEDTYPE SEALES- PVC AO STEE AT 2BrC T4H 9A0D05S 9(AZ3i) 0bI BYI f CELLSOETCAL TOTHE AbDVE STCOREIDNAEP• NOUS-TTYE SHELTE• AT TIDEWATERPiVEP EDGE NRL REPORT 6715 9 The initial activation of these cells is invariably accompanied by a terminal voltage which in the first several seconds following activation may be more than 100% above the subsequent steady-state value. The effect persists strongly for as much as 10 to 15 sec- onds. That is, after 10 to 15 seconds the voltage may yet be 60 to 75% above a later 1-hour or steady-state value, depending on the battery load resistance, age, and previous env~ron- mental history, and materials plus coaistruction. It is thought to be due to momentary depolarization of the cathode by surface oxides and/or dissolved oxygen in the electrolyte. For those special applications requiring triggering energy upon activation with sea water, the inert-cathode/Mg-anode cell has demonstrated adequate energy storage capacity to fire explosive squibs, which in turn power a subsequent mechanical or hydraulic actuation mechanism. Equally important to the needs at hand is the ability to retain this capability over months of exposed ready-to-use standby or storage. Although cells of this type are intrinsically more resistant to exposed storage dete- rioration than the silver chloride type of sea-water battery, they are not impervious to aging. The particular aging of concern here is the reduction in ability to supply energy quickly upon activation. Surface contamination could be of a nature as to inhibit electro- chemical action and quick impulse response for a period of seconds or minutes, yet even- tually clear away for good steady-state performance. The impulse cell is more sensitive to aging 4-n the longer term cells. To g.n some notion of the effect of exposed aging on impulse action, quantities of batteries were placed in both controlled and exposed environments, and samples were withdrawn periodically for measurement and recording of voltage and energy to a specified load. The results of the energy measurements are shown in Fig. 10. Since one important application requirement for these cells called for squib firing within a 3-second interval after immersion, the energy points apply solely to the 3-second time following activation. The recorded data from which these points are taken, however, include energy (i.e., inte- grated power) and instantaneous voltage up to several minutes. From this more general data picture, it was observed that over a 5 to 10 second interval energy output always exceeded requirements. The straight lines of Fig. 10 approximate the lower bounds of 3-second energy for both storage conditions. ,s0o 12'• I s•' 1 234b ,r, 0304050100 ELAPSED STORAGE TIME IN WEE-(S Fig. 10 - Impulse battery performance. The energy was released into a 3-ohm Ioa during a 3-second interval. o2 - OI-LOWEST ACCEPTABLE ENERGY \ 0o i 0 L_•A, pm .rh&IS2•oDAYS ,:,j••..PDF Image | INERT-CATHODE SEA-WATER BATTERY
PDF Search Title:
INERT-CATHODE SEA-WATER BATTERYOriginal File Name Searched:
AD0673399.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |