
PDF Publication Title:
Text from PDF Page: 009
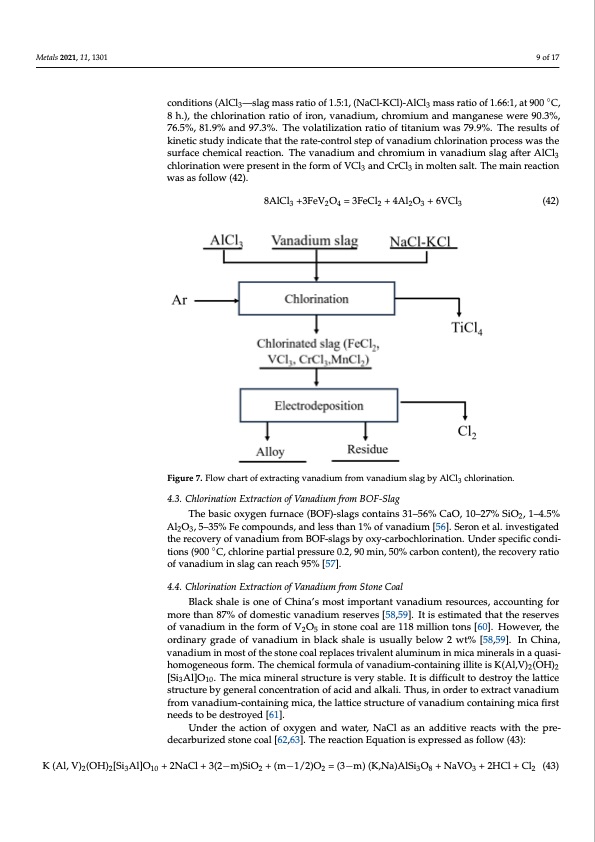
Metals 2021, 11, x FOR PEER REVIEW 10 of 18 Metals 2021, 11, 1301 9 of 17 reaction temperature, reaction time, mass ratio of AlCl3/slag and mass ratio of salt/AlCl3 on the chlorination ratio of valuable elements were investigated. Under optimal chlorina-◦ conditions (AlCl —slag mass ratio of 1.5:1, (NaCl-KCl)-AlCl mass ratio of 1.66:1, at 900 C, tion conditions (AlC3 l3—slag mass ratio of 1.5:1, (NaCl-KCl)-A3lCl3 mass ratio of 1.66:1, at 8 h.), the chlorination ratio of iron, vanadium, chromium and manganese were 90.3%, 900 °C, 8 h.), the chlorination ratio of iron, vanadium, chromium and manganese were 76.5%, 81.9% and 97.3%. The volatilization ratio of titanium was 79.9%. The results of 90.3%, 76.5%, 81.9% and 97.3%. The volatilization ratio of titanium was 79.9%. The results kinetic study indicate that the rate-control step of vanadium chlorination process was the of kinetic study indicate that the rate-control step of vanadium chlorination process was surface chemical reaction. The vanadium and chromium in vanadium slag after AlCl the surface chemical reaction. The vanadium and chromium in vanadium slag after AlCl3 3 chlorination were present in the form of VCl3 and CrCl3 in molten salt. The main reaction chlorination were present in the form of VCl3 and CrCl3 in molten salt. The main reaction was as follow (42). was as follow (42). 8AlCl3 +33FFeeV2OO4 ==3F3eFCeCl2l+ 4+A4lA2Ol 3O+ 6+VC6Vl3Cl 3242233 Figure 7. Flow chart of extracting vanadium from vanadium slag by AlCl3 chlorination. Figure 7. Flow chart of extracting vanadium from vanadium slag by AlCl3 chlorination. 4.3. Chlorination Extraction of Vanadium from BOF-Slag 4.3. Chlorination Extraction of Vanadium from BOF-Slag (42)(42) The basic oxygen furnace (BOF)-slags contains 31–56% CaO, 10–27% SiO2, 1–4.5% The basic oxygen furnace (BOF)-slags contains 31–56% CaO, 10–27% SiO2, 1–4.5% Al2O3, 5–35% Fe compounds, and less than 1% of vanadium [56]. Seron et al. investigated Al2O3, 5–35% Fe compounds, and less than 1% of vanadium [56]. Seron et al. investigated the recovery of vanadium from BOF-slags by oxy-carbochlorination. Under specific condi- ◦ the recovery of vanadium from BOF-slags by oxy-carbochlorination. Under specific con- tions (900 C, chlorine partial pressure 0.2, 90 min, 50% carbon content), the recovery ratio ditions (900 °C, chlorine partial pressure 0.2, 90 min, 50% carbon content), the recovery of vanadium in slag can reach 95% [57]. ratio of vanadium in slag can reach 95% [57]. 4.4. Chlorination Extraction of Vanadium from Stone Coal 4.4. Chlorination Extraction of Vanadium from Stone Coal Black shale is one of China’s most important vanadium resources, accounting for more than 87% of domestic vanadium reserves [58,59]. It is estimated that the reserves Black shale is one of China’s most important vanadium resources, accounting for of vanadium in the form of V O in stone coal are 118 million tons [60]. However, the more than 87% of domestic vanad2ium5 reserves [58,59]. It is estimated that the reserves of ordinary grade of vanadium in black shale is usually below 2 wt% [58,59]. In China, vanadium in the form of V2O5 in stone coal are 118 million tons [60]. However, the ordi- vanadium in most of the stone coal replaces trivalent aluminum in mica minerals in a quasi- nary grade of vanadium in black shale is usually below 2 wt% [58,59]. In China, vanadium homogeneous form. The chemical formula of vanadium-containing illite is K(Al,V) (OH) in most of the stone coal replaces trivalent aluminum in mica minerals in a quasi-hom2 o- 2 [Si Al]O . The mica mineral structure is very stable. It is difficult to destroy the lattice geneo3us f1o0rm. The chemical formula of vanadium-containing illite is structure by general concentration of acid and alkali. Thus, in order to extract vanadium K(Al,V)2(OH)2[Si3Al]O10. The mica mineral structure is very stable. It is difficult to destroy from vanadium-containing mica, the lattice structure of vanadium containing mica first the lattice structure by general concentration of acid and alkali. Thus, in order to extract needs to be destroyed [61]. vanadium from vanadium-containing mica, the lattice structure of vanadium containing Under the action of oxygen and water, NaCl as an additive reacts with the pre- mica first needs to be destroyed [61]. decarburized stone coal [62,63]. The reaction Equation is expressed as follow (43): Under the action of oxygen and water, NaCl as an additive reacts with the pre-decar- burized stone coal [62,63]. The reaction Equation is expressed as follow (43): K (Al, V)2(OH)2[Si3Al]O10 + 2NaCl + 3(2−m)SiO2 + (m−1/2)O2 = (3−m) (K,Na)AlSi3O8 + NaVO3 + 2HCl + Cl2 (43)PDF Image | Extraction of the Rare Element Vanadium
PDF Search Title:
Extraction of the Rare Element VanadiumOriginal File Name Searched:
metals-11-01301.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |