
PDF Publication Title:
Text from PDF Page: 005
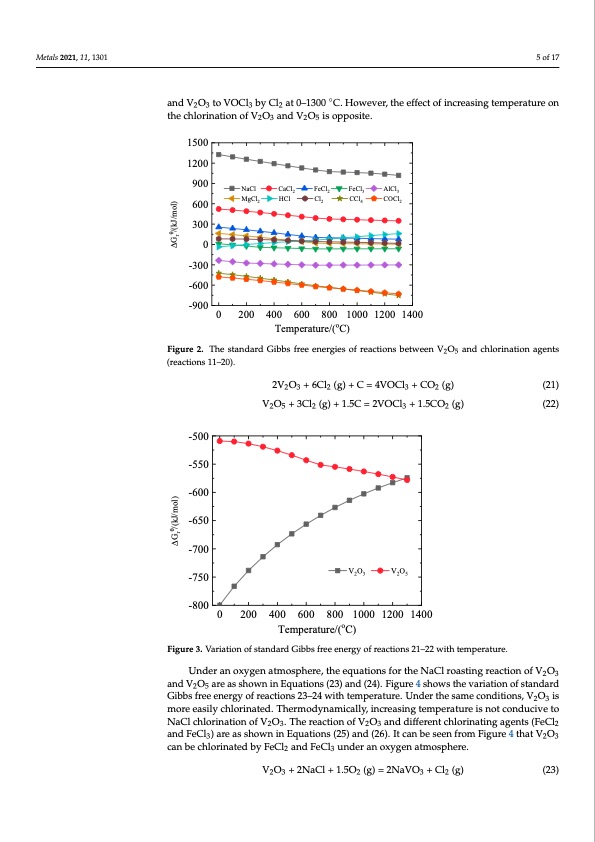
Metals 2021, 11, 1301 5 of 17 V2O5 + 6HCl (g) = 2VOCl3 + 3H2O (g) V2O5 + 3COCl2 (g) = 2VOCl3 + 3CO2 (g) V2O5 + 1.5CCl4 (g) = 2VOCl3 + 1.5CO2 (g) V2O5 + 3Cl2 (g) = 2VOCl3 + 1.5O2 (g) (17) (18) (19) and V2O3 to VOCl3 by Cl2 at 0–1300 ◦C. However, the effect of increasing temperature on the chlorination of V2O3 and V2O5 is opposite. 1500 1200 900 600 300 0 -300 -600 -900 0 200 400 600 800 1000 1200 1400 Temperature/(oC) Figure 2. The standard Gibbs free energies of reactions between V2O5 and chlorination agents Figure 2. The standard Gibbs free energies of reactions between V2O5 and chlorination agents (reac- (20) NaCl CaCl2 FeCl2 FeCl3 AlCl3 MgCl2 HCl Cl2 CCl4 COCl2 (reactions 11–20). tions 11–20). Metals 2021, 11, x FOR PEER REVIEW 2V2O3 + 6Cl2 (g) + C = 4VOCl3 + CO2 (g) 6 of 18 (21) The V2O5 and V2O3 reacting with C and Cl2 in the temperature range from 0 °C to 1300 °C are expressed asVf2oOllo5 w+s3iCnl2Eq(gu)a+tio1n.5sC(2=1)2aVnOdC(2l32+). F1i.5gCurOe23(sgh)ows standard Gibb(2s2) free energies of reactions 21–22 at 0–1300 °C. Adding C realizes the chlorination of V2O5 and V2O3 to VOCl3 by Cl2 at 0–1300 °C. However, the effect of increasing temperature on O2 (g) (21) -500 tion of V2O3 and V2O5 is opposite. 2V2O3 +6Cl2 (g)+C=4VOCl3 +C V2O5 + 3Cl2 (g) + 1.5C = 2VOCl3 + 1. V2O3 V2O5 the chlorina -550 -600 -650 -700 -750 5CO2 (g) (22) -800 0 200 400 600 800 1000 1200 1400 Temperature/(oC) Figure 3. Variation of standard Gibbs free energy of reactions 21–22 with temperature. Figure 3. Variation of standard Gibbs free energy of reactions 21–22 with temperature. Under an oxygen atmosphere, the equations for the NaCl roasting reaction of V2O3 Under an oxygen atmosphere, the equations for the NaCl roasting reaction of V2O3 andVO areasshowninEquations(23)and(24).Figure4showsthevariationofstandard and V2O5 2are5as shown in Equations (23) and (24). Figure 4 shows the variation of standard GibGbisbfbresefreeneregnyeorfgryeaocftiroenasc2t3io–n24s w23it–h2t4emwpitehrateumre.pUernadteurrteh.eUsanmdercotnhdeitsiaomnse, Vc2oOn3ditions, V2O3 is is moorreeeaesailsyilcyhclohrlinoartienda.tTehde.rTmhoedrymnaomdiycanllaym, inicarellays,iningcteremapseinragtutremispneortactounrdeuicsivneot conducive to to NaCl chlorination of V2O3. The reaction of V2O3 and different chlorinating agents (FeCl2 NaCl chlorination of V2O3. The reaction of V2O3 and different chlorinating agents (FeCl2 and FeCl3) are as shown in Equations (25) and (26). It can be seen from Figure 4 that V2O3 and FeCl3) are as shown in Equations (25) and (26). It can be seen from Figure 4 that V2O3 can be chlorinated by FeCl2 and FeCl3 under an oxygen atmosphere. can be chlorinated by FeCl2 and FeCl3 under an oxygen atmosphere. V2O3 + 2NaCl + 1.5O2 (g) = 2NaVO3 + Cl2 (g) (23) V2O3 + 2NaCl + 1.5O2 (g) = 2NaVO3 + Cl2 (g) (24) (23) 2V2O5 + 4NaCl + O2 (g) = 4NaVO3 + 2Cl2 (g) (25) (26) According to the above thermodynamic analysis, the valence state of vanadium, re- V2O3 + 2FeCl3 + O2 (g) = 2VOCl3 (g) + Fe2O3 V2O3 + 3FeCl2 + 1.75O2 (g) = 2VOCl3 (g) + 1.5Fe2O3 ΔGrθ/(kJ/mol) ΔGrθ/(kJ/mol)PDF Image | Extraction of the Rare Element Vanadium
PDF Search Title:
Extraction of the Rare Element VanadiumOriginal File Name Searched:
metals-11-01301.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |