
PDF Publication Title:
Text from PDF Page: 004
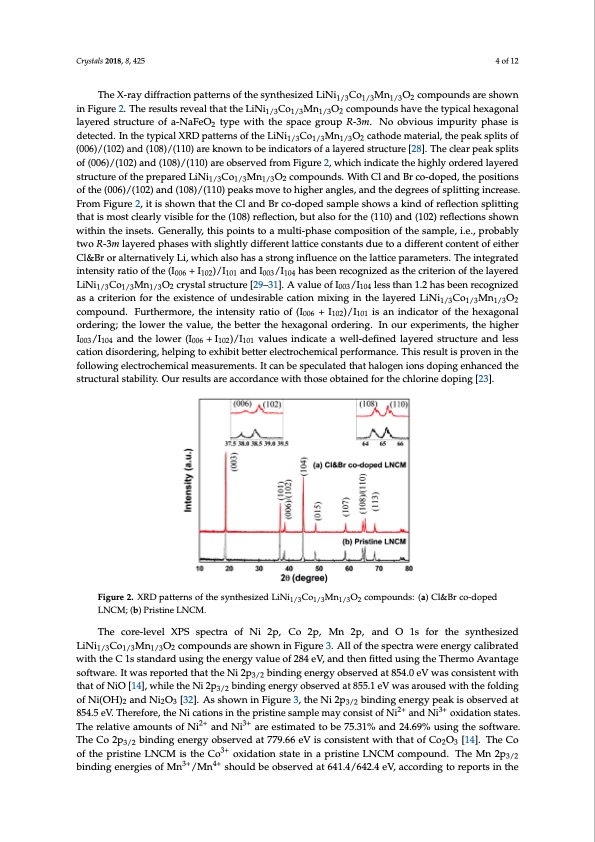
Crystals 2018, 8, 425 4 of 12 The X-ray diffraction patterns of the synthesized LiNi1/3Co1/3Mn1/3O2 compounds are shown in Figure 2. The results reveal that the LiNi1/3Co1/3Mn1/3O2 compounds have the typical hexagonal layered structure of a-NaFeO2 type with the space group R-3m. No obvious impurity phase is detected. In the typical XRD patterns of the LiNi1/3Co1/3Mn1/3O2 cathode material, the peak splits of (006)/(102) and (108)/(110) are known to be indicators of a layered structure [28]. The clear peak splits of (006)/(102) and (108)/(110) are observed from Figure 2, which indicate the highly ordered layered structure of the prepared LiNi1/3Co1/3Mn1/3O2 compounds. With Cl and Br co-doped, the positions of the (006)/(102) and (108)/(110) peaks move to higher angles, and the degrees of splitting increase. From Figure 2, it is shown that the Cl and Br co-doped sample shows a kind of reflection splitting that is most clearly visible for the (108) reflection, but also for the (110) and (102) reflections shown within the insets. Generally, this points to a multi-phase composition of the sample, i.e., probably two R-3m layered phases with slightly different lattice constants due to a different content of either Cl&Br or alternatively Li, which also has a strong influence on the lattice parameters. The integrated intensity ratio of the (I006 + I102)/I101 and I003/I104 has been recognized as the criterion of the layered LiNi1/3Co1/3Mn1/3O2 crystal structure [29–31]. A value of I003/I104 less than 1.2 has been recognized as a criterion for the existence of undesirable cation mixing in the layered LiNi1/3Co1/3Mn1/3O2 compound. Furthermore, the intensity ratio of (I006 + I102)/I101 is an indicator of the hexagonal ordering; the lower the value, the better the hexagonal ordering. In our experiments, the higher I003/I104 and the lower (I006 + I102)/I101 values indicate a well-defined layered structure and less cation disordering, helping to exhibit better electrochemical performance. This result is proven in the following electrochemical measurements. It can be speculated that halogen ions doping enhanced the structural stability. Our results are accordance with those obtained for the chlorine doping [23]. Figure 2. XRD patterns of the synthesized LiNi1/3Co1/3Mn1/3O2 compounds: (a) Cl&Br co-doped LNCM; (b) Pristine LNCM. The core-level XPS spectra of Ni 2p, Co 2p, Mn 2p, and O 1s for the synthesized LiNi1/3Co1/3Mn1/3O2 compounds are shown in Figure 3. All of the spectra were energy calibrated with the C 1s standard using the energy value of 284 eV, and then fitted using the Thermo Avantage software. It was reported that the Ni 2p3/2 binding energy observed at 854.0 eV was consistent with that of NiO [14], while the Ni 2p3/2 binding energy observed at 855.1 eV was aroused with the folding of Ni(OH)2 and Ni2O3 [32]. As shown in Figure 3, the Ni 2p3/2 binding energy peak is observed at 854.5 eV. Therefore, the Ni cations in the pristine sample may consist of Ni2+ and Ni3+ oxidation states. The relative amounts of Ni2+ and Ni3+ are estimated to be 75.31% and 24.69% using the software. The Co 2p3/2 binding energy observed at 779.66 eV is consistent with that of Co2O3 [14]. The Co of the pristine LNCM is the Co3+ oxidation state in a pristine LNCM compound. The Mn 2p3/2 binding energies of Mn3+/Mn4+ should be observed at 641.4/642.4 eV, according to reports in thePDF Image | Enhanced High Voltage Performance of Chlorine Bromine
PDF Search Title:
Enhanced High Voltage Performance of Chlorine BromineOriginal File Name Searched:
crystals-08-00425.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |