
PDF Publication Title:
Text from PDF Page: 019
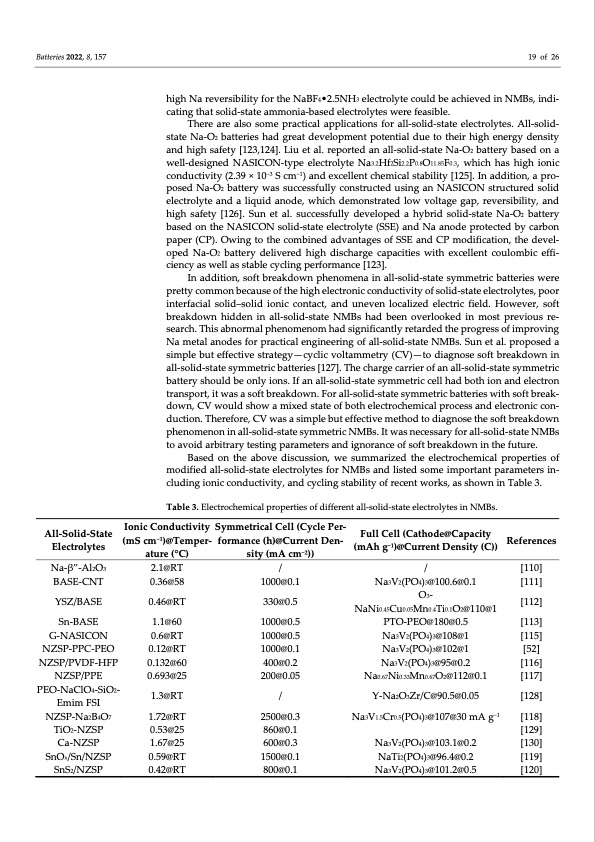
Batteries 2022, 8, 157 19 of 26 high Na reversibility for the NaBF4•2.5NH3 electrolyte could be achieved in NMBs, indi‐ cating that solid‐state ammonia‐based electrolytes were feasible. There are also some practical applications for all‐solid‐state electrolytes. All‐solid‐ state Na‐O2 batteries had great development potential due to their high energy density and high safety [123,124]. Liu et al. reported an all‐solid‐state Na‐O2 battery based on a well‐designed NASICON‐type electrolyte Na3.2Hf2Si2.2P0.8O11.85F0.3, which has high ionic conductivity (2.39 × 10−3 S cm−1) and excellent chemical stability [125]. In addition, a pro‐ posed Na‐O2 battery was successfully constructed using an NASICON structured solid electrolyte and a liquid anode, which demonstrated low voltage gap, reversibility, and high safety [126]. Sun et al. successfully developed a hybrid solid‐state Na‐O2 battery based on the NASICON solid‐state electrolyte (SSE) and Na anode protected by carbon paper (CP). Owing to the combined advantages of SSE and CP modification, the devel‐ oped Na‐O2 battery delivered high discharge capacities with excellent coulombic effi‐ ciency as well as stable cycling performance [123]. In addition, soft breakdown phenomena in all‐solid‐state symmetric batteries were pretty common because of the high electronic conductivity of solid‐state electrolytes, poor interfacial solid–solid ionic contact, and uneven localized electric field. However, soft breakdown hidden in all‐solid‐state NMBs had been overlooked in most previous re‐ search. This abnormal phenomenom had significantly retarded the progress of improving Na metal anodes for practical engineering of all‐solid‐state NMBs. Sun et al. proposed a simple but effective strategy—cyclic voltammetry (CV)—to diagnose soft breakdown in all‐solid‐state symmetric batteries [127]. The charge carrier of an all‐solid‐state symmetric battery should be only ions. If an all‐solid‐state symmetric cell had both ion and electron transport, it was a soft breakdown. For all‐solid‐state symmetric batteries with soft break‐ down, CV would show a mixed state of both electrochemical process and electronic con‐ duction. Therefore, CV was a simple but effective method to diagnose the soft breakdown phenomenon in all‐solid‐state symmetric NMBs. It was necessary for all‐solid‐state NMBs to avoid arbitrary testing parameters and ignorance of soft breakdown in the future. Based on the above discussion, we summarized the electrochemical properties of modified all‐solid‐state electrolytes for NMBs and listed some important parameters in‐ cluding ionic conductivity, and cycling stability of recent works, as shown in Table 3. Table 3. Electrochemical properties of different all‐solid‐state electrolytes in NMBs. All‐Solid‐State Electrolytes Na‐β”‐Al2O3 BASE‐CNT YSZ/BASE Sn‐BASE G‐NASICON NZSP‐PPC‐PEO NZSP/PVDF‐HFP NZSP/PPE PEO‐NaClO4‐SiO2‐ Emim FSI NZSP‐Na2B4O7 TiO2‐NZSP Ca‐NZSP SnOx/Sn/NZSP SnS2/NZSP Ionic Conductivity Symmetrical Cell (Cycle Per‐ (mScm−1)@Temper‐ formance(h)@CurrentDen‐ Full Cell (Cathode@Capacity (mAh g−1)@Current Density (C)) / Na3V2(PO4)3@100.6@0.1 O3‐ NaNi0.45Cu0.05Mn0.4Ti0.1O2@110@1 PTO‐PEO@180@0.5 Na3V2(PO4)3@108@1 Na3V2(PO4)3@102@1 Na3V2(PO4)3@95@0.2 Na0.67Ni0.33Mn0.67O2@112@0.1 Y‐Na2O3Zr/C@90.5@0.05 Na3V1.5Cr0.5(PO4)3@107@30 mA g−1 Na3V2(PO4)3@103.1@0.2 NaTi2(PO4)3@96.4@0.2 Na3V2(PO4)3@101.2@0.5 References [110] [111] [112] [113] [115] [52] [116] [117] [128] [118] [129] [130] [119] [120] ature (°C) 2.1@RT 0.36@58 0.46@RT 1.1@60 0.6@RT 0.12@RT 0.132@60 0.693@25 1.3@RT 1.72@RT 0.53@25 1.67@25 0.59@RT 0.42@RT sity (mA cm−2)) / 1000@0.1 330@0.5 1000@0.5 1000@0.5 1000@0.1 400@0.2 200@0.05 / 2500@0.3 860@0.1 600@0.3 1500@0.1 800@0.1PDF Image | Electrolyte Engineering for Sodium Metal Batteries
PDF Search Title:
Electrolyte Engineering for Sodium Metal BatteriesOriginal File Name Searched:
batteries-08-00157.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |