
PDF Publication Title:
Text from PDF Page: 002
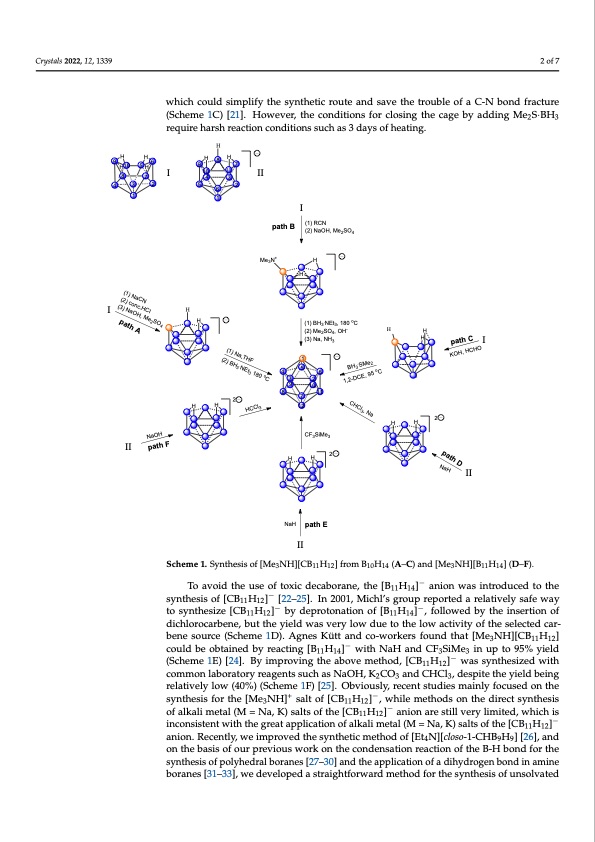
022, 12, x FOR PEER REVIEW 2 of 8 Crystals 2022, 12, 1339 replacing toxic cyanide to form the initial C-B bond (Scheme 1B) [20]. Later, the group of Kennedy used formaldehyde and decaborane to form the B-C bond, which could simplify the synthetic route and save the trouble of a C-N bond fracture (Scheme 1C) [21]. How- 2 of 7 which could simplify the synthetic route and save the trouble of a C-N bond fracture ever, the conditions for closing the cage by adding Me2S·BH3 require harsh reaction con- (Scheme 1C) [21]. However, the conditions for closing the cage by adding Me2S·BH3 ditions such as 3 days of heating. require harsh reaction conditions such as 3 days of heating. H 9HH6H8H H105H I 9 117 II 10 87 43 432 562 1 I 1 Me3N+ I H 1 456 32 path B (1) RCN (2) NaOH, Me2SO4 H (1) BH3.NEt3, 180 oC (2) Me2SO4, OH- H (3) Na, NH3 H H H H 1 2 II I 7 HH 12 2 9 10 8 11 HH II CF3SiMe3 2 II HH NaH path E Scheme 1. SynStchheesmiseo1f.[SMyne3tNheHsi]s[oCfB[1M1He31N2]Hfr]o[CmBB111H0H121]4 f(rAo–mCB)1a0nHd14[M(Ae–3CN)Han][dB[1M1He134N] (HD][–BF1)1.H14] (D–F). To avoid the use of toxic decaborane, the [B H ]− anion was introduced to the − 1114 To avoid the use of toxic decaborane, the [B11H14] anion was introduced to the syn- synthesis of [CB11H12]− [22–25]. In 2001, Michl’s group reported a relatively safe way thesis of [CB11H12]− [22–25]. In 2001, Michl’s group reported a relatively safe way to syn- to synthesize [CB11H12]− by deprotonation of [B11H14]−, followed by the insertion of thesize [CB11H12]− by deprotonation of [B11H14]−, followed by the insertion of dichlorocar- dichlorocarbene, but the yield was very low due to the low activity of the selected car- bene, but the yield was very low due to the low activity of the selected carbene source bene source (Scheme 1D). Agnes Kütt and co-workers found that [Me3NH][CB11H12] (Scheme 1D). Agnes Kütt and co-workers found th−at [Me3NH][CB11H12] could be obtained could be obtained by reacting [B11H14] with NaH and CF3SiMe3 in up to 95% yield −− by reacting [(BS1c1hHe1m4]ew1Eit)h[2N4a].HBaynidmCprFo3vSinMget3hienaubpovtoe 9m5e%thyodie,ld[C(BSchHem]e 1wEa)s[2s4y]n.tBhyesimze-d with − proving the acboomvmeomneltahbodra,t[oCryB1r1eHag12e]nwtsassucshynatshNesaiOzeHd, KwiCthOcoamndmConHClalb,odraetsoprityertehaegyeineltds being 233 relatively low (40%) (Scheme 1F) [25]. Obviously, recent studies mainly focused on the such as NaOH, K2CO3 and CHCl3, despite the yield being relatively low (40%) (Scheme synthesis for the [Me NH]+ salt of [CB H ]−, while methods on the direc+t synthesis 1F) [25]. Obviously, recent studie3s mainly focused11on12the synthesis for the [Me3NH] salt of alkali metal (M = Na, K) salts of the [CB H ]− anion are still very limited, which is − 11 12 of [CB11H12] , while methods on the direct synthesis of alkali metal (M = Na, K) salts of the − inconsistent with the great application of alkali metal (M = Na, K) salts of the [CB11H12]− [CB11H12] anion are still very limited, which is inconsistent with the great application of anion. Recently, we improved the synthetic method of [Et4N][closo-1-CHB9H9] [26], and alkali metal (M = Na, K) salts of the [CB11H12]− anion. Recently, we improved the synthetic on the basis of our previous work on the condensation reaction of the B-H bond for the method of [Et4N][closo-1-CHB9H9] [26], and on the basis of our previous work on the con- synthesis of polyhedral boranes [27–30] and the application of a dihydrogen bond in amine densation reaction of the B-H bond for the synthesis of polyhedral boranes [27–30] and boranes [31–33], we developed a straightforward method for the synthesis of unsolvated the application of a dihydrogen bond in amine boranes [31–33], we developed a straight- forward method for the synthesis of unsolvated potassium and sodium salts of the 11 12 2 path A (1) NaCN (2) conc.HCl (3) NaOH, Me2SO4 NaH path C KOH, HCHO (1) Na,THF (2) BH3.NEt3 180 oC 1,2-DCE, 95 oC CHCl3, Na HCCl3 NaOH BH3.SMe2 path F path DPDF Image | Efficient Way to Directly Synthesize Unsolvated Alkali Metal
PDF Search Title:
Efficient Way to Directly Synthesize Unsolvated Alkali MetalOriginal File Name Searched:
crystals-12-01339.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Salgenx
Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our flow battery manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |